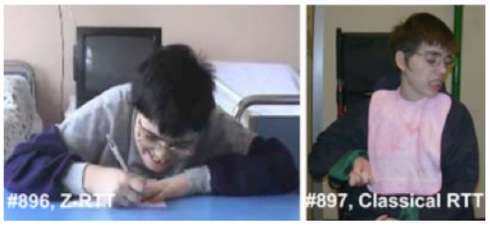
You are currently browsing the tag archive for the ‘MECP2’ tag.

by Carol Morton
Three years ago, a study showed that a bone marrow transplant performed in pre-symptomatic male mice models of Rett Syndrome substantially extended their lives and reduced symptoms of disease. The unexpected findings caught the attention of Rett researchers, physicians, and parents.
Seeking to validate the results and therefore strengthen the case for clinical studies, four other research groups launched their own mouse studies. In independent experiments, each lab was unable to replicate the original findings.
The researchers combined their results into a single paper, published May 20 in Nature, the same journal that published the original positive results. The new study is posted online only as a “brief communication arising,” a category for new scientific data that challenge the findings of an original research paper in the journal, according to Veronique Kiemer, executive editor of the Nature Publishing Group.
The new paper means that bone marrow transplants may not be a viable therapeutic option, but the pair of papers could point the way to new insights into Rett and ways to fix it, said Monica Carson, a neuroimmunologist at the University of California, Riverside, who was not involved in any of the studies.
“The key is that both answers are possible,” Carson said. “ It’s important to figure out the differences between the papers.”
The original paper came from the lab of Jonathan Kipnis, a neuroimmunologist at University of Virginia in Charlottesville. Kipnis and his colleagues explore the role of the immune system in healthy and diseased brains. No stranger to controversial findings, he has shown that T cells closely surrounding the brain are somehow crucial to normal cognitive function.
In fact, the team first conducted the transplants to test the idea that inadequate T cells in Rett mice might explain their cognitive impairment. “We proved our original hypothesis wrong,” Kipnis said. But with new immune cells, the mice lived much longer. Most cases of Rett can be traced to a malfunctioning gene on the X chromosome called methyl-CpG-binding protein 2 (MeCP2). A transplant fixed the faulty gene in the mice’s immune cells.
How was a new immune system exerting a protective effect? A clue came from stem cell transplant studies for Alzheimer’s disease, where another kind of circulating immune cell, called monocytes or macrophages, lodge in the brain and clear away debris that may cause neurodegeneration. After further experiments, Kipnis and his co-authors proposed that monocytes with good MeCP2 genes also migrated to the brain in the Rett models and helped their brain-dwelling microglia cousins in some unknown way.
Last month, the Kipnis team reported the first molecular and cellular evidence that MeCP2 controls gene expression in macrophages and that some types of macrophages in the brain and throughout the body may be especially vulnerable early in disease. “This work is a beautiful example of how the immune and nervous systems are intimately associated, sharing common molecular pathways and potentially affecting the function of one another in many dynamic ways,” according to a commentary published with the paper.
The original paper was funded by RSRT. Given the serious nature and risk of a bone marrow transplant, RSRT felt it was crucial to reproduce the findings before supporting any clinical trials. RSRT awarded funding to Andrew Pieper, now at the University of Iowa, who had provided the original mice for the Kipnis study, and Antonio Bedalov, at the Fred Hutchinson Cancer Research Center, a scientist and oncologist, who works with bone marrow transplant patients.
Independently, Peter Huppke, at University Medical Center Gottingen in Germany, and Jeffrey Neul, now at University of California, San Diego, also attempted replications. None of the four labs saw the significant effects seen by Kipnis.
For his part, Kipnis interprets the new paper differently. “Most importantly, it confirms our initial findings, although not as dramatically,” he said, pointing to a small increased lifespan effect that could be seen if two figures were combined. He and two lead co-authors of the original paper have written a detailed response in the comment section for the paper. It outlines suspected issues with the new paper, including mice that may have inadvertently acquired a mixed genetic background and may therefore have a version of graft versus host disease (GVHD) which would have confounded the results. This would be analogous to a person receiving bone marrow that was not a tissue match. Bedalov denied that possibility, saying GVHD would be obvious because the mice would have additional symptoms.
The first mouse study had prompted clinical investigators to add boys with Rett to a hematopoietic stem cell transplant protocol last year. Boys with classic Rett mutations have more severe disease and usually die by age 2. Based on the new findings, the trial has withdrawn Rett as a disease eligibility, wrote principal investigator Weston Miller at the University of Minnesota in an email. No boys with Rett had enrolled in the trial.
Other researchers contacted by RSRT applaud the attempt to replicate the bone marrow transplant findings before considering clinical trials, but they see a more important unfolding story is the role of the immune system in Rett disease biology.
Looking ahead, “discrepancies between labs do occur,” Kipnis and his co-authors wrote, “and understanding the cause of varying results can ultimately lead to an even better understanding of the scientific or disease-related process in question.”
Every cell in our body contains the same genes. Yet a brain cell is distinctly different from a heart cell or a liver cell. What differentiates these cells are the genes that are either silenced or active and the degree of activation of the genes, also known as expression.
Scientists have known for many years that the “Rett protein”, MeCP2, regulates the expression of other genes. The big question has been, which genes?
Michael Greenberg of Harvard University, and his lab members Harrison Gabel and Benyam Kinde, may have an answer: long genes. The journal, Nature, is publishing this finding today.
Genes are made up of nucleotides (think back to high school biology: A,T,C,G) The average gene has about 20,000 nucleotides, but some have as many as a million. The scientists in the Greenberg lab found that the MeCP2 protein acts as a dimmer switch, dampening the expression of long genes. When the MeCP2 protein is absent, as in the case of Rett, with no dimmer switch to regulate it, long gene expression goes up. Any deviation from the normal expression pattern causes problems.
From this finding, the scientists suggest that Rett Syndrome may be caused by a widespread overexpression of long genes.
You may be asking yourself, why does this matter? It matters because there is a drug that can rebalance the expression levels of long genes. The Greenberg lab has already tested this drug in cells missing the MeCP2 protein with encouraging results. Experiments are now underway to test the drug in Rett mice.
This is a promising development. We are providing the following resources to help you understand the progress being reported today.
Animation of Findings
Interview with Greenberg Lab Members
For a variety of reasons the pharmaceutical industry over the last few years has become more and more interested in rare disease. This is great news for Rett Syndrome. As terms like orphan drug designation, breakthrough therapy, efficacy, drug approval, market exclusivity become part of our everyday lingo it is important that our understanding of them is based in facts. As we start the process of learning and sharing information we bring you this interview with regulatory consultant and Former Director of the FDA Office of Orphan Product Development, Timothy Coté.
Guest Blogger Beth Jones, whose daughter Jocelyn has Rett Syndrome, urges more families to take action
Yesterday, we sat in 5 hours of traffic taking Jocelyn to Los Angeles for her orthopedic appointment. Her scoliosis is turning her into a question mark, her back brace is so uncomfortably tight it disturbs her g-tube and makes feedings difficult. We strive to do the best for our daughter but we are constantly juggling details like this. After the long day, the-oh-too-familiar feelings of “defeat” and “alone” swarmed over me. A feeling I am certain every Rett Syndrome parent feels from time to time.
But today is a new day. Today I am preparing for our first committee meeting of the year for Jocelyn’s Journey. Today, I get to fight back and drink up the hope that once Jocelyn is cured, days like yesterday will be a memory, instead of my day to day reality.
This is our 5th year hosting Jocelyn’s Journey and we’ve proudly supported the Rett Syndrome Research Trust with 100% of the revenue from each event. As I reflect on our first year hosting, I was a mess, honestly. It was hard for me to admit that I needed help. I was so afraid no one would buy tickets to come to our event. I was worried what people would think of me, asking for money. I was worried my friends and family would be too busy with their own lives to join forces with me and make a committee. I have learned, in the past five years, that I couldn’t have been more wrong.
The outpouring of people who support us each year has humbled us. I’ve learned that people want to help us, they just don’t know how. Having an annual event that supports the research that will one day CURE Jocelyn inspires and thrills everyone to help. Our committee has grown from 5 people to 20 in just a few short years! We sell out of tickets each and every year for the event, donations roll in and we are becoming well known around our community. RSRT is very helpful each and every year and has guided us on what to do, as I had NEVER done anything like this before. Year one, left me hooked—I figured out how to get over the sadness, defeat, feeling alone and helpless: I fight back! Jocelyn’s Journey’s moto: “No donation is too small or too large” and we stand on that! We are grateful for every dollar and I know RSRT is too!
A few months ago, I was speaking to Tim Freeman, Program Director at RSRT, and he expressed to me “if just 10 more families would do what you are doing with fundraising, it would change the research”. I was sad that money was standing in the way of Jocelyn and all Rett girls getting treatment. But then I was excited—this was in our control! We, all Rett families, can fix this problem and expedite treatment!
The Jocelyn’s Journey committee and I have set a goal this year to be one of those 10 families by doubling our average fundraising earnings. So that leaves 9 families! There has to be someone out there who has thought of hosting an event but has been worried about how to get started. I relate to the hesitation, but be assured, you will have more support and guidance than you would expect. Call Monica or Tim, please! An event is absolutely worth it and brings in the most funding. An event can be anything you want it to be—a barbecue, a poker party, a pancake breakfast, a 5K walk or run. And there are other things you can do too. I did an “informational” booth at church to talk about Jocelyn and without asking, people handed over donations! Be creative, be fun! If all 15,000 Rett families hosted something small that raised just a few hundred to a thousand dollars, the impact would be huge. No event is too large, or too small! Please learn from my fear in the beginning: your friends and family want to help you—they just need you to give them the opportunity to help.
 As Rett parents we know, the first step is the hardest one. Some of us, like myself, are still waiting to see their girl take her first step. The same holds true for fundraising—getting started is the first step and the hardest part. Once you get going though, you don’t want to stop. I won’t stop, I will not give up. I don’t expect Jocelyn to give up and I owe her the same strength in return.
As Rett parents we know, the first step is the hardest one. Some of us, like myself, are still waiting to see their girl take her first step. The same holds true for fundraising—getting started is the first step and the hardest part. Once you get going though, you don’t want to stop. I won’t stop, I will not give up. I don’t expect Jocelyn to give up and I owe her the same strength in return.
by Monica Coenraads
As always at RSRT, our funded projects are aimed at developing effective treatments and a cure for Rett Syndrome. But one of the key roadblocks to achieving this has been a lack of knowledge about the MeCP2 protein and how it functions. In 2011 RSRT decided to conduct an experiment of our own. Take three world-class laboratories and give them the necessary financial resources ($5.5 million awarded to date) and infrastructure to tackle a question that no one yet has been able to answer: what does the MeCP2 protein do?
Almost four years later the labs of Gail Mandel (Oregon Health and Science University), Michael Greenberg (Harvard University), and Adrian Bird (University of Edinburgh) are getting closer to that answer and have made the following discoveries along the way— discoveries that could prove to be invaluable to how we will ultimately change the lives of girls and women afflicted with Rett:
- It was known that MeCP2 binds to DNA in brain cells, but the Consortium showed that MeCP2 has a binding partner, called NCOR, that is known to silence genes. Importantly, the Consortium showed that mutations that disrupt the ability of MeCP2 to bind to NCOR are associated with Rett in people, thus lending support for the essential nature of this interaction.
- MeCP2 is modulated by phosphorylation for normal nervous system function.
- The Consortium has shown that gene therapy can reverse symptoms in symptomatic female Rett mice. This work is being actively followed up by a dedicated “Gene Therapy Consortium” also funded by RSRT.
- As yet unpublished work is shedding light on the crucial question of which genes in the brain are controlled by MeCP2. It may be possible to target these genes via specific drugs.

The MECP2 Consortium meets in Boston twice a year and holds conference calls in between the meetings. The meetings at first included only Professors Mandel, Bird and Greenberg but have grown over time to include many of the lab members. The middle and right pictures are from the last meeting in October 2014.
Recently I posed a few questions to the three investigators about the important work they are tackling.
 Despite much effort, there is little consensus among scientists regarding what MeCP2 actually does in the brain. Needless to say it helps greatly when fixing something to know exactly what has gone wrong, so this is an issue that badly needs addressing. Fortunately the research tools for getting at the problem have gotten much better over the past few years and we are now in a good position to nail this problem down.
Despite much effort, there is little consensus among scientists regarding what MeCP2 actually does in the brain. Needless to say it helps greatly when fixing something to know exactly what has gone wrong, so this is an issue that badly needs addressing. Fortunately the research tools for getting at the problem have gotten much better over the past few years and we are now in a good position to nail this problem down.
 It’s important to know why the loss of MeCP2 gives rise to Rett as well as helping to determine a minimally active form that might be better suited to gene replacement approaches.
It’s important to know why the loss of MeCP2 gives rise to Rett as well as helping to determine a minimally active form that might be better suited to gene replacement approaches.
 It is hard for me to imagine a treatment for Rett that isn’t based on an understanding of MeCP2 function. Based on what we already know about MeCP2 it is clear that it’s function in neurons is quite complex and difficult to understand. That together with the complexity of the brain makes me think it is unlikely that a therapy that isn’t based on a deep understanding of MeCP2 function is likely to work. Nevertheless, I wouldn’t rule it out.
It is hard for me to imagine a treatment for Rett that isn’t based on an understanding of MeCP2 function. Based on what we already know about MeCP2 it is clear that it’s function in neurons is quite complex and difficult to understand. That together with the complexity of the brain makes me think it is unlikely that a therapy that isn’t based on a deep understanding of MeCP2 function is likely to work. Nevertheless, I wouldn’t rule it out.
 If we could correct the genetic changes that cause MeCP2 to dysfunction in Rett so that the defective gene is replaced by a healthy one, then we would not need to know how MeCP2 works. This ideal scenario is becoming less of a fantasy, but is still some ways from being a reality. Knowing precisely what pathways MeCP2 regulates offers the prospect of treating downstream effects of the mutation as an alternative to correcting the gene. It is too early to say at the moment which approach is more likely to bear fruit so it is important to try both.
If we could correct the genetic changes that cause MeCP2 to dysfunction in Rett so that the defective gene is replaced by a healthy one, then we would not need to know how MeCP2 works. This ideal scenario is becoming less of a fantasy, but is still some ways from being a reality. Knowing precisely what pathways MeCP2 regulates offers the prospect of treating downstream effects of the mutation as an alternative to correcting the gene. It is too early to say at the moment which approach is more likely to bear fruit so it is important to try both.
 I think investigators in other disciplines would love to have what we have built together. The Consortium is a wonderful stimulus for new ways of thinking critically about how to study and/or cure Rett. Two heads, or in this case three heads, are always better than one, particularly because we have different expertise and backgrounds. And we can build on each other’s discoveries much more quickly.
I think investigators in other disciplines would love to have what we have built together. The Consortium is a wonderful stimulus for new ways of thinking critically about how to study and/or cure Rett. Two heads, or in this case three heads, are always better than one, particularly because we have different expertise and backgrounds. And we can build on each other’s discoveries much more quickly.
 The Consortium is a new way of working that has benefited our lab’s work greatly. Being able to thrash out ideas and explore different ways of looking at Rett with top class scientists from different backgrounds has sharpened up everybody’s research. All the partners have fully committed to the Consortium idea and as a result no one feels inhibited about robustly questioning the others. This kind of free and frank exchange keeps us on our toes and always makes research better. As well as ideas and data, we share materials and equipment, which speeds up our work and reduces costs.
The Consortium is a new way of working that has benefited our lab’s work greatly. Being able to thrash out ideas and explore different ways of looking at Rett with top class scientists from different backgrounds has sharpened up everybody’s research. All the partners have fully committed to the Consortium idea and as a result no one feels inhibited about robustly questioning the others. This kind of free and frank exchange keeps us on our toes and always makes research better. As well as ideas and data, we share materials and equipment, which speeds up our work and reduces costs.
 Science is usually built on a competitive model where PIs compete for funding and try to make and publish discoveries ahead of their peers. Sharing data and plans for experiments with people who were once competitors is a different way of working – but one that is also liberating. It requires trust and a recognition by everyone that a higher goal is at stake. This Consortium really works. Hopefully we are poised to advance our knowledge of MeCP2 in ways that will make a difference therapeutically.
Science is usually built on a competitive model where PIs compete for funding and try to make and publish discoveries ahead of their peers. Sharing data and plans for experiments with people who were once competitors is a different way of working – but one that is also liberating. It requires trust and a recognition by everyone that a higher goal is at stake. This Consortium really works. Hopefully we are poised to advance our knowledge of MeCP2 in ways that will make a difference therapeutically.
 It has been very rewarding. Nothing really has surprised me because I knew Adrian Bird and Mike Greenberg pretty well beforehand and I had ultimate confidence in the high quality of their science and their collegiality.
It has been very rewarding. Nothing really has surprised me because I knew Adrian Bird and Mike Greenberg pretty well beforehand and I had ultimate confidence in the high quality of their science and their collegiality.
Participating in the Consortium and working collaboratively with the Mand el and Bird labs has been a wonderful experience. The rigor and pace of scientific progress is much greater with the three labs working together than would be possible if each lab were working alone. Monica has been essential to keeping the Consortium on target and helping make sure the scientists in the Consortium continue to work together effectively over time.
el and Bird labs has been a wonderful experience. The rigor and pace of scientific progress is much greater with the three labs working together than would be possible if each lab were working alone. Monica has been essential to keeping the Consortium on target and helping make sure the scientists in the Consortium continue to work together effectively over time.
The lab members from the three labs have thoughts of their own about the MECP2 Consortium.
| Consortium Research Projects | Reflections on Meeting | |
| Harrison Gabel (Greenberg lab)  |
Benyam Kinde, Caitlin Gilbert, William Renthal and myself have been studying how MECP2 functions when it is bound to DNA in neurons and how it might control the levels of many proteins important for the function of neurons in the brain. This exciting work may provide an answer to the long-standing question of exactly what goes wrong in individual neurons in the Rett Syndrome brain when MeCP2 is lost. I described recent results from experiments using cultured mouse neurons that lack MeCP2 to test whether drugs can correct the defects in these neurons. Promising results from these experiments suggest that a drug can at least partially correct these defects. We are now beginning to explore if this drug can improve symptoms in mice with Rett Syndrome by delivering the drug to the brain of these mice. | In general it is truly unprecedented to have three powerhouse labs that work on the mechanism of MeCP2 get together for a meeting and share their most recent data. The reality is that under any other circumstances we would be competing (hopefully in a congenial way!) and largely keeping secrets from one another until the data were published. This Consortium breaks down these walls and as a result the science moves much faster. I commend Adrian, Gail, and Mike for being willing to share so much, all of the lab members for trusting in the other Consortium members to treat them fairly, and most of all RSRT for creating such a unique and effective Consortium. Thanks! |
| Benyam Kinde (Greenberg lab)  |
At the meeting I spoke about experiments that provide insight into the mechanism of MeCP2-mediated gene regulation. Through a series of biochemical, genetic and genomic experiments, I described how DNA methylation, specifically occurring in the CA dinucleotide sequence context in neurons, serves as a critical site for MeCP2 binding and regulation of gene expression in the developing brain. | The Consortium has provided a unique opportunity to share novel findings, which ultimately has led to invaluable discussions that provide critical insight into the design and interpretation of experiments. In this way, the Consortium has allowed all three laboratories to develop projects at an exceedingly rapid pace. |
| Matt Lyst (Greenberg lab)  |
Last year we published evidence for a model where the primary function of MeCP2 is to recruit the NCoR/SMRT co-repressor complex to chromatin. At the last Consortium meeting I presented work aimed at further testing this hypothesis, and also investigating which components of this complex are most relevant to Rett Syndrome. |
Sharing current data between labs means we all receive input from people in the field but outside of our own labs at a much earlier stage than would normally happen. |
| Sabine Lagger (Bird lab)  |
MeCP2 is classically described as a methyl DNA binding protein exerting its function by exclusively binding to methylated CpG dinucleotides. It became obvious in recent years that MeCP2 can not only bind to methyl CpG dinucleotides but has been suggested to bind to other forms of modified DNA in in vitro experiments broadening its DNA binding sites. My work aims at establishing in vivo models to analyze MeCP2 binding patterns in brain cells. I therefore sort neuronal and glial cells from mouse brain and subject them to DNA methylation analysis to the single base pair resolution level. I can then overlay these maps with MECP2 binding profiles and identify the true in vivo MeCP2 targets. This analysis will help us to understand how MeCP2 is acting on chromatin and what the necessary signal for its binding are. | I was invited to the RSRT Consortium meetings in Boston twice and both times I could not wait to get back to the lab and start working again. The possibility to present and discuss my work with like- minded and enthusiastic experts on MeCP2 is extremely beneficial and made me look at scientific problems from different angles. Meeting Rett Syndrome patients’ parents was very interesting for me and made me realize even more how important it is to keep working on understanding this devastating disease and to ultimately find a cure. |
| John Connelly (Bird lab)  |
The MeCP2 protein acts by interacting with DNA at many locations inside cells. It is not clear however exactly what DNA sequences MeCP2 binds to on chromosomes. My work aims to identify what these sequences are. My hope is that understanding how the protein works in greater detail will aid the design of an effective therapeutic strategy. |
I was really pleased to be able to attend the recent MeCP2 Consortium meeting in Boston as it was really nice to meet and talk to the parents of children with Rett syndrome and discuss my work with them and the other scientists present. When in Boston I found that other members of the Consortium had, reassuringly, reached similar conclusions and this gave me the impetus to continue my particular avenue of investigation. |
| Hume Akahori-Stroud (Greenberg lab)  |
I talked about a series of experiments on understanding the role of DNA methylation patterning in the brain. DNA methylation is a chemical modification of DNA that is abundant in neurons, and regulates MeCP2 function. Understanding the molecular mechanisms of DNA methylation in regulating MeCP2 is important to understand how MeCP2 works. | It was great getting to know what other laboratories were up to, and I think the meeting has increased my understanding on MeCP2 a step further. |
| John Sinnamon (Mandel lab)  |
Many of the mutations in MeCP2, which cause Rett Syndrome are single nucleotide changes known as point mutations. Our goal is to harness the catalytic activity of an enzyme already found in cells to target and correct these mutations in MeCP2 RNA. We have been able to edit MeCP2 RNA in vitro and are working towards testing our strategy in a mouse containing a point mutation, which has been identified in several Rett patients. | Attending the RSRT Consortium meetings is a wonderful experience. There is a collaborative atmosphere you do not see at large scientific meetings and everyone is focused on understanding the biology of MeCP2 so that we can understand Rett Syndrome. For me personally, it is very powerful to meet parents of girls with Rett and to talk to them about my research. It provides a reminder of what I am working towards and I think gives the families an opportunity to talk one on one with the scientists they support. |
| Ruth Shah (Bird lab)  |
My project involves modeling Rett – causing mutations in human neurons. Model systems are a great way to elucidate the molecular mechanisms behind diseases and to understand how a protein works in a cellular context. I really hope these human neurons will help us to understand the details involved in Rett, they may even provide a useful tool for testing gene therapy ideas in! | Being part of the Consortium meeting gave me the opportunity to meet neuroscientists and gain advice and ideas from them on how to improve my project and my research. The flexibility to present my project in detail to an experienced audience without fear of my project being torn apart is a great thing. It provides the freedom for open chat and encouragement and an exchange of thoughts and ideas in a positive manner, rather than having a competitive undertone to the day. This is the environment that is needed in scientific research to encourage advances in knowledge. It allows for collaboration in a productive manner, for example as a result of the Consortium, I now have a list of genes whose expression I should look into from one of the other attending labs. If it weren’t for the Consortium I doubt information like this would be shared among labs in such an open manner. |
| Jackie Guy (Bird lab)  |
Using information we have about the MECP2 mutations found in girls with Rett we have been able to identify two important regions of the protein: the region that binds to methylated DNA (MBD) and a small region which binds to a repressor complex, NCoR/SMRT. I am producing a number of different mutations in mouse embryonic stem cells in order to investigate why they cause Rett Syndrome. This may lead to a better understanding of the function and/or structure of MeCP2. | I enjoyed hearing about the work of the other two groups in the Consortium. Each group has its own particular view of what MeCP2 is doing and I found it refreshing to think about things from a slightly different angle. |
| Rebekah Tillotson (Bird lab)  |
Missense mutations that cause Rett are almost all located in either the region of MeCP2 protein that binds to methylated DNA or the region that interacts with the NCoR/SMRT repressor complex. This suggests that the function of MeCP2 is to form a ‘bridge’ between chromatin and the repressor proteins, and loss of this bridge results in brain dysfunction in Rett. I am testing this hypothesis by manipulating the MeCP2 gene in mice, and then carrying out behavioral tests to determine whether they exhibit the symptoms observed in the mouse models of Rett. | The RSRT Consortium was a great opportunity for me to meet other scientists in the field, to learn about and discuss their work, and to get valuable input on my own project. The informality and openness of the discussion made it a thoroughly rewarding and stimulating experience. |
| Kyla Brown (Bird lab)  |
Rett Syndrome severity varies partly because of the nature of the MECP2 mutation. My project focuses on making animal models of “milder” mutations to see if there are specific functions of MeCP2 that these mutations affect. | The Consortium provides a unique opportunity to communicate findings within a group of expert researchers as well as to forge collaborations. I enjoyed being able to appreciate others’ perspectives on the same clinical and biological problem and seeing how this can result in advances in the MeCP2 field. |
| Martha Koerner (Bird lab)  |
I am working on MeCP2 duplication syndrome. I am trying to understand what happens if you do have too much MeCP2 and what we can do to counteract the symptoms caused by excess MeCP2. | The Consortium meeting in October was the first one I’ve attended. I’ve found it incredibly helpful to be able to talk to other scientists who work on the same gene, to learn about novel findings of others that will impact my research and also to get input from experts into the work I’m doing. |
| Susan Su (Greenberg lab)  |
I am interested in examining the ultrastructural changes underlying the altered cellular morphology and synaptic connections of a mouse model of Rett Syndrome. | I enjoy our lively, intellectual discussions at the Consortium meetings where we all share a common goal of gaining a deeper understanding of MeCP2. The Consortium meetings are wonderful opportunities to reflect on preliminary data and to share helpful reagents and insights for our experiments. |
| Jim Selfridge (Bird lab)  |
My work in the Bird Lab focuses on the production and analyses of genetically modified animal models of Rett. These models have proved invaluable to Rett research over the years and the novel models continue to increase our understanding of MeCP2 function and the underlying molecular basis of Rett. I am also committed to using these Rett models to investigate potential therapeutic strategies. | Although I never actually presented any of my research in person at the last meeting I was still able to benefit hugely by attending. The Consortium meetings and in particular the relaxed, open and friendly format provide a great focus for Rett researchers. It gives us a perfect opportunity to have our work critically assessed by experts in the field, even in the early stages of a project. This often affords us extra insight that we might not get from the sometimes insular environment of our own individual groups. I look forward to being part of many more meetings! |
| Will Renthal (Greenberg lab)  |
Rett is characterized by profound synaptic dysfunction. I am studying the role MeCP2 plays in coordinating the gene programs responsible for normal synaptic responses to neuronal activity. Specifically, our laboratory has found that neuronal activity drives the rapid phosphorylation of MeCP2 at serine 86, so my current efforts are aimed at identifying the functional significance of this event. | I think the Consortium was a fantastic opportunity to share ideas with people from a variety of backgrounds to accelerate Rett research. We were having technical difficulties with some of our experiments and the collective wisdom of the Consortium has been crucial for overcoming them. |
| Justyna Cholewa-Waclaw (Bird lab)  |
The aim of my project is to define primary transcriptional consequences of MeCP2 depletion. In order to do that I use an in vitro system based on immortalized human neural precursors which can be differentiated into dopaminergic neurons. I generated cells with reduced amount of MeCP2, entirely depleted MeCP2 and increased levels of MeCP2. Gene expression changes in these cells with different levels of MeCP2 will be studied additionally in the context of gene body methylation and hydroxymethylation to provide the molecular basis of MeCP2 function. | I think the Consortium meetings are great. The informal nature is very beneficial. I had brilliant opportunity to discuss my work with people working on the same problem. I could also ask questions more openly and know what other people are doing. |
So many of our kids suffer from gut problems – constipation, reflux, bloating and pain. Despite the prevalence of GI issues in Rett this is an area that has been mostly unexplored by scientists. So we are happy to add Dr. Ali Khoshnan of Caltech to our growing list of funded researchers. Dr. Khoshan will be exploring the gut physiology of mice models of Rett. He will also be testing a powerful probiotic (not currently available for people) in the mice to see if any Rett symptoms improve. Watch the video below to learn how the study of the microbiome (the community of microorganisms that populate us and outnumber our cells 10:1) has become a very hot field in science and how it might be applied to Rett Syndrome.
by Monica Coenraads
I am delighted to give you a brief update on the MECP2 Gene Therapy Consortium, the collaboration of four elite labs that RSRT launched earlier this year. As you know, the Consortium is charged with developing gene therapy techniques that could treat or significantly reverse the symptoms of Rett. Our goal is to get to clinical trials. The project is grounded in work done last year by Consortium members Gail Mandel and Brian Kaspar that showed for the first time reversal of Rett symptoms in mice using gene therapy techniques that have the potential to be used in humans. The reversal of symptoms in mice was quite remarkable, but there are many challenges to translating that to a reversal in girls and women with Rett. The Consortium is attacking these challenges head on.
Earlier this month members of the Consortium met in the boardroom of a JFK Airport hotel in New York (we did not want to waste any of our meeting time traveling to and from a hotel in Manhattan). In addition to Gail Mandel, other members of the Consortium are Stuart Cobb (University of Glasgow), Steven Gray (University of North Carolina at Chapel Hill), and Brian Kaspar (Nationwide Children’s Hospital). The Consortium has a timeline of 3 years and a budget of $1.5 million. RSRT hosts in-person Consortium meetings twice a year as well as regularly scheduled conference calls.

From left moving clockwise:
Sarah Sinnet (Gray lab), Steve Gray, Brian Kaspar, Stuart Cobb, Saurabh Garg (Mandel lab), Kamal Gadalla (Cobb lab).
Guest participant Ruth Shah (Bird lab) joined the meeting by Skype as did Gail Mandel and Mark Bailey.

The advantages gained by labs working collaboratively are clear: speed (four labs contributing to the work that has to be done), real time sharing of information means more brainpower and broader perspectives for problem solving. This is an obvious example of more heads are better than one.
Three facts make Rett Syndrome an attractive disease for gene therapy: it is monogenic; it is remarkably reversible in animal models; delivering MECP2 does not require understanding its function.
There are several hurdles to overcome. There is a requirement for MECP2 in every part of the brain so the gene will need to be broadly delivered. Also, the MECP2 Duplication Syndrome suggests that too much MECP2 is bad. It is difficult in gene therapy to regulate how many copies of a gene enter a cell and how much protein is made so the issue of MECP2 dosage must be carefully explored. We know that having too much MECP2 from conception and through early development causes serious symptoms. But does the same hold true if extra MECP2 is delivered later in life? Also, is it possible that females tolerate greater amounts of this protein than males? These questions must be answered before a clinical trial can be proposed.
 Consortium members are also working on the following key issues:
Consortium members are also working on the following key issues:
1) Vector optimization – The vector is the Trojan horse that delivers the gene into a cell. There are many types of vectors in use and many more under development. For Rett we need a vector that can get into the brain and spread efficiently throughout the organ. The delivery route will affect the vector of choice. For example, if you deliver intravenously (via the blood stream) there is concern that a large amount of vector will end up in the liver potentially causing toxicity. To get around this problem a vector that de-targets the liver would be very useful. If dosage of MECP2 turns out to be problematic vectors that can be turned off will be required.
2) MEPC2 optimization – There are limits to the amount of DNA that can be packaged into a vector. The entire MECP2 gene does not fit. Scientists therefore have to select the parts of the gene they think are the most important. In essence they need to design a “mini-MECP2 gene’. Similar “mini-gene” work is also underway in the lab of Adrian Bird and will be shared with the Consortium.
3) Delivery route optimization – Gene therapy can be delivered via the blood stream, intrathecally into the spinal cord (like an epidural), or directly to the brain. Each route has its own advantages and disadvantages.
4) Optimizing how much gene therapy to deliver – the scientists are delivering low, medium and high dosages in an attempt to see how much is needed to get a therapeutic effect without generating toxic side effects.
We thank our precious donors who make this critical research possible!
In Their Own Words

It is very stimulating to be part of such a focused group of experts on gene therapy approaches towards Rett. The previous studies that we performed in collaboration with the Kaspar group were promising in showing that expression of a good copy of MeCP2, delivered systemically with AAV9, ameliorated Rett-like symptoms in female mice and prolonged survival significantly in affected males. Most surprisingly, but importantly, although we did not achieve a large amount of expression of the good MeCP2 in brains of the treated mice, we still saw behavioral benefits. We are now trying to improve the expression level of delivered MeCP2 by redesigning the vector, according to ideas and experimental results presented at the Consortium meetings. The openness of the investigators propels our studies and makes for a productive venture that would not be possible by any one individual laboratory. Additionally, it saves time because we can move on from doing obvious experiments that were done already in another laboratory. Finally, for those crucial experiments that had positive results, we have the ability to reproduce them in a different laboratory to insure that the results are solid.
– Gail Mandel
 The Kaspar Laboratory is extremely excited about the potential to deliver gene therapies to the CNS. We are encouraged with our delivery studies to target cells efficiently in the brain, where one requires the proper expression of MECP2. Furthermore, our clinical trial in Spinal Muscular Atrophy has to date demonstrated the safety of this gene therapeutic in children which is excellent news for development of gene therapeutics in diseases, such as Rett. As a laboratory, we have bolstered our Rett efforts and are making great progress in testing the safety and developing the pre-clinical data necessary for developing a treatment. Our approach is building off the success of our collaboration with Dr. Gail Mandel. We are thankful for her continued support on our steep learning curve of Rett. This Consortium allows us to learn from each other’s studies. It’s a great group of scientists and I’m privileged to be a part of it. I see the progress we are collectively making and the commitment to the development of a therapy for Rett patients. The path is starting to look much clearer to get there.
The Kaspar Laboratory is extremely excited about the potential to deliver gene therapies to the CNS. We are encouraged with our delivery studies to target cells efficiently in the brain, where one requires the proper expression of MECP2. Furthermore, our clinical trial in Spinal Muscular Atrophy has to date demonstrated the safety of this gene therapeutic in children which is excellent news for development of gene therapeutics in diseases, such as Rett. As a laboratory, we have bolstered our Rett efforts and are making great progress in testing the safety and developing the pre-clinical data necessary for developing a treatment. Our approach is building off the success of our collaboration with Dr. Gail Mandel. We are thankful for her continued support on our steep learning curve of Rett. This Consortium allows us to learn from each other’s studies. It’s a great group of scientists and I’m privileged to be a part of it. I see the progress we are collectively making and the commitment to the development of a therapy for Rett patients. The path is starting to look much clearer to get there.
– Brian Kaspar
 The Cobb lab shares Brian’s excitement about the consortium’s efforts and the potential for gene therapy to counteract the root cause of Rett Syndrome. The project is progressing on multiple fronts from vector design/optimization to assessing best delivery methods and testing for efficacy and safety. Whilst the concept of gene therapy is a very simple one, the route to developing a safe and effective therapy is not at all straightforward. A key element of the consortium is that it enables us to share ideas and to discuss and act on emerging results from the four labs in real time. This will inevitably lead to more rapid progress in addressing the various challenges. As well as coordinating efforts, the consortium also enables us to cross validate key experiments to ensure findings are robust and reproducible across laboratories. After our Consortium meeting Kamal and I traveled to visit Steve Gray’s lab at UNC Chapel Hill. It was an extremely valuable few days as we were able to not only observe but also practice various delivery route methods. We also were able to compare and standardize how we score neurological features seen in the mice. Spending time together also provided an opportunity to further discuss vector development. Our trip to the US for both the Consortium meeting and visit to UNC was very productive.
The Cobb lab shares Brian’s excitement about the consortium’s efforts and the potential for gene therapy to counteract the root cause of Rett Syndrome. The project is progressing on multiple fronts from vector design/optimization to assessing best delivery methods and testing for efficacy and safety. Whilst the concept of gene therapy is a very simple one, the route to developing a safe and effective therapy is not at all straightforward. A key element of the consortium is that it enables us to share ideas and to discuss and act on emerging results from the four labs in real time. This will inevitably lead to more rapid progress in addressing the various challenges. As well as coordinating efforts, the consortium also enables us to cross validate key experiments to ensure findings are robust and reproducible across laboratories. After our Consortium meeting Kamal and I traveled to visit Steve Gray’s lab at UNC Chapel Hill. It was an extremely valuable few days as we were able to not only observe but also practice various delivery route methods. We also were able to compare and standardize how we score neurological features seen in the mice. Spending time together also provided an opportunity to further discuss vector development. Our trip to the US for both the Consortium meeting and visit to UNC was very productive.
– Stuart Cobb
 Our efforts to treat Rett syndrome are built on 7 years of experience with the Rett community along with “bench to bedside” approaches that we are taking for six other inherited diseases. Our gene therapy clinical trial for Giant Axonal Neuropathy laid an important foundation for a similar approach to be taken with Rett syndrome. Gene therapy for Rett is an enormous challenge, but the last few years have garnered a great deal of excitement based on the similar positive findings published by all 4 laboratories in this consortium in 2 seminal papers. We are excited to be part of this group, and together we can accomplish much more than my lab alone. When Dr. Cobb visited our lab recently he provided critical expertise in a short visit that saved us an enormous amount of time and effort if we had been working alone. This is a small example of the many benefits we have had from working together in a collaborative fashion.
Our efforts to treat Rett syndrome are built on 7 years of experience with the Rett community along with “bench to bedside” approaches that we are taking for six other inherited diseases. Our gene therapy clinical trial for Giant Axonal Neuropathy laid an important foundation for a similar approach to be taken with Rett syndrome. Gene therapy for Rett is an enormous challenge, but the last few years have garnered a great deal of excitement based on the similar positive findings published by all 4 laboratories in this consortium in 2 seminal papers. We are excited to be part of this group, and together we can accomplish much more than my lab alone. When Dr. Cobb visited our lab recently he provided critical expertise in a short visit that saved us an enormous amount of time and effort if we had been working alone. This is a small example of the many benefits we have had from working together in a collaborative fashion.
– Steve Gray
Dear Friends,
I’ve been thinking a lot lately about the phenomenon of the Ice Bucket Challenge that swept the nation this past summer. This was a major coup for research on ALS, also known as Lou Gehrig’s disease. By most accounts it resulted in more than $100 million going to several organizations that support ALS research. It also created a whole new level of awareness of the disease. I was very happy to see this. ALS is an awful disease and research to find treatments or a cure is highly worthy of support. It was also great to see the country so caught up in a movement for a good cause.
A number of people have asked me if I wished the whole phenomenon had focused on Rett Syndrome instead of ALS. My answer—of course I do. How could I not? I have a daughter who struggles every moment of every day with Rett. What would I not do to hear her say “daddy” or “mommy” or to watch her walk across a room? It’s not just my daughter who moves me to say this; it’s also the more than 150 other girls and women with Rett Syndrome I’ve now met. It’s their faces and their expressive and intelligent eyes that keep me awake at night. So, yes, I wish the Ice Bucket Challenge had raised $100 million for Rett research to change their lives and my daughter’s life. That kind of funding would create a sea change for our research. It would greatly speed up progress. It would buy more time from the scientists who are already working so hard on Rett Syndrome; it would allow us to expand the number of researchers focusing on Rett; it would enable more projects and more scientific collaboration. In short, it would very likely have a tremendous impact on accelerating us towards what we all want so badly—effective treatments and a cure.I could go on wishing, but that wouldn’t be very productive. So instead I started thinking about what I learned from the Ice Bucket Challenge and how I might apply it. I asked myself why people gave to this phenomenon so readily and generously. It’s clear that they weren’t giving because of information they got on ALS or the research. Most of the Ice Bucket videos I saw contained little or no information on ALS. So why did people give? I think they gave because of who asked them. They gave because they were “challenged” by someone they knew, trusted, and respected—a friend, a colleague, their daughter or son, their parents, or an old college or high school pal. Even celebrities gave because they were “challenged” by some other celebrity they know or work with.
The Ice Bucket Challenge showed me that what’s all important is who is doing the asking. It showed me something that sounds basic, but is so important—that each of us has the power to ask and that we all can act on it with our own friends and family and make a difference to the research and to our daughters’, granddaughters’, sisters’, and nieces’ lives. And now, around the holiday season as the year is ending and people are thinking about their giving, is the best time to do it. I know I’m making it sound easier than it is. Asking people for money, even for a compelling and promising cause like ours, is hard. But what I find is that the results are surprising and rewarding—not just the fact that people give, but that they give so happily and that they are so pleased to be making a difference.
You might say to me—well that’s nice; but how do I ask? What do I say? Do I do it in person or in a letter or email? Those are all good questions. But there’s no one-size-fits-all answer. My advice is to tell your story about why you care about this, and then ask if people will give to this cause that matters so much to you. You know best what would work for your friends and family—maybe it’s a heartfelt letter or email; maybe it’s knocking on a few doors; maybe it’s taking a group of friends out for coffee or a drink. There’s no right or wrong way to do it. Keep it simple, be honest, respectful, and do it from the heart.
So this holiday season, if you’re not already involved with events or asking family and friends for support of the research, try it. Try asking a few people and see what kind of response you get. I think you’ll be pleasantly surprised. I am here to help if you want to think it through or run a letter by me. Please do not hesitate to call or email me any time. Thank you so much.
Tim Freeman
Monica Coenraads interviews Michael Green, MD, PhD of the UMASS School of Medicine about his newly published paper in Proceedings of the National Academy of Sciences. The work was funded, in part, by RSRT. He has identified a number of genes that when disrupted can reactivate the silenced X chromosome in females. Some of these genes lie in pathways that are druggable which makes this work potentially clinically relevant not only for Rett Syndrome but also for other X-linked disorders.
Prof. Green’s paper was covered by SFARI.org. in an article written by Jessica Wright.
Rousing silenced X chromosome may treat Rett syndrome
Drugs that activate the silent copy of the X chromosome in women may be able to undo the damage from mutations in genes located there. The study, published 2 September in Proceedings of the National Academy of Sciences, offers hope for treating Rett syndrome and other disorders linked to the chromosome1.
One copy of the two X chromosomes women carry is randomly silenced in each cell of the body. This occurs when the chromosome makes small pieces of RNA, called X-inactive specific transcript, or Xist. A cloud of Xist coats the chromosome and blocks its expression.
Female mice lacking Xist die in utero, so X inactivation was thought to be required for survival. The new study suggests otherwise.
The researchers identified 13 genes required for X inactivation. Female mice missing STC1, one of these genes, show expression of genes from both copies of X and have no obvious symptoms.
“The mouse findings suggest that you might be able to survive without X chromosome inactivation,” says lead researcher Michael Green, professor of molecular medicine at the University of Massachusetts Medical School.
Discovering drugs to treat diseases of the central nervous system is a formidable task. Our brains are easily the most complex machines on the planet and the more we learn about this machine, the more daunting seems the task of fixing it when things go wrong. This point is brought home for me when meeting people with some of the CNS disorders I hope to help treat. I’m new to Rett Syndrome but have now been to a couple of events along with girls suffering Rett and their families. How can a mutation in a single gene result in such a complex outcome?! There is a specific answer to this question, because MeCP2 mutation causes Rett, a specific disease. Yet it is a long and winding road from MeCP2 to that characteristic hand wringing and the many other symptoms Rett girls experience over a lifetime. Where to step in with a drug therapy and what to expect as an outcome?
The best way to deal with such complex drug discovery problems is head on – identify the root cause of a disorder, fix that, and relief from even the most complex symptoms should follow. For Rett, there is very good basic science data to suggest that if we restore MeCP2 function then we will restore brain function, and so with habilitation therapy achieve a cure. Encouraging research along this line is ongoing and we can hope for success.
However, many, if not most, of the drug discovery efforts around Rett syndrome target the consequences of MeCP2 mutations to provide a therapeutic benefit. Put another way, these approaches aim to mend the damage done to the brain that results from lack of functional MeCP2. In fact, this is the most common approach in CNS drug discovery, where root causes are seldom clear. Such approaches can be quite effective. Perhaps the best example is in Parkinson’s disease, where dopamine neurons in the brain die off. We don’t know why dopamine neurons die or how to prevent this die off, that is, how to fix the root cause of Parkinson’s. But we have a pretty good understanding of the dopamine system and from this knowledge drug therapies, and more recently deep brain stimulation therapies, have been developed that compensate for the loss of dopamine neurons. Take a look at this YouTube video of the effect of deep brain stimulation in a Parkinson’s patient. Clearly, this patient is being enormously helped by his therapy, even though the therapy does not fix the root cause of dopamine neuron loss.
Can we fix the Rett brain and anticipate that complex symptom relief will follow? I think the answer is ‘yes’. And, there is a guidepost on how we may go about this in the efforts being made to discover new therapies to treat cognitive defects in schizophrenia. Schizophrenia, like Rett, is a neurodevelopmental disorder that results in an incredibly complex and variable array of symptoms. Of these, the cognitive deficits are the most debilitating, keeping patients out of school and work even after their more florid symptoms are under control. But these cognitive deficits are complex, as are their outcomes (that’s an understatement!). How would we know that a new therapy had a meaningful effect in the face of such complexity, let alone how to prove a benefit in a Phase II clinical trial? The schizophrenia research community, NIMH, FDA, and the pharmaceutical industry, took this problem on and the result was the MATRICS and CNTRICS Initiatives. In MATRICS, complex global cognitive deficits in schizophrenia were broken down into simpler components like working memory, for example as measured in the ability to remember a short word list. The idea is that improving a patient’s working memory, coupled with habilitation therapy, would make the patients better able to keep track of tasks and so hold a job or finish school. CNTRICS takes this one reductionist strategy one step further by trying to identify the fundamental physiological brain abnormalities that underlie the simpler cognitive components. For example, associating an EEG abnormality with a working memory deficit. The logic now is that, if we fix the EEG abnormality, we will fix working memory, and the patient has an improved cognitive toolbox to use in daily life. Clinical trial follows this progression – first, fix the EEG abnormality, which is most quantifiable and requires the smallest number of patients; second, take the learnings on drug dosing etc. from the EEG trial to prove a reliable drug regimen that fixes working memory; third, use this drug regimen, in combination with habilitation, to explore the potential for ‘real life’ benefits.
I think a structured approach following a MATRICS/CNTRICS model could be very impactful in developing drug therapies for Rett. But the devil is in the details. The most obvious and immediate questions are scientific- what to measure first, second, third? Less obvious but no less important are questions around clinical development strategies and timelines for such an approach. Where along such a path does such a drug become approved and so commercially available? These latter issues will require significant thought and discussion among all in the Rett community. More of my thoughts on the science and these broader issues in future posts.
by Monica Coenraads
Anyone who knows anything about Rett Syndrome knows that the disorder is primarily seen in girls. The disorder is caused by disruption of the MECP2 gene located on the X chromosome. Girls have two X chromosomes one with the disrupted gene and one with the healthy gene. Having some healthy MeCP2 protein allows girls to survive but at the expense of severe impairment that comes with Rett.
Since boys only have the one X chromosome they have no healthy MECP2 at all. These boys typically have a more severe form of the disease and often die in early childhood. (There are genetic situations that allow boys to present like classic girls with Rett, for example if they have Klinefelter Syndrome which gives them two Xs.)
The fact that boys only have one X is the reason most often given for why Rett is seen in girls. However this is not accurate. While having the sole X is the reason boys often succumb to the disease it is NOT the reason why Rett is primarily a woman’s disease. That reason has to do with where the MECP2 mutation originates.
Many studies over the past decade have provided evidence that the vast majority of MECP2 mutations originate in the sperm. Since fathers give an X to their daughters and a Y chromosome to their sons the MECP2 mutation can only be transmitted from father to daughter. This is the reason why Rett is seen primarily in girls.
Boys, on the other hand, get their MECP2 mutations from their mother, a situation that arises only rarely. (Mutations can also originate in a single cell as the male embryo is developing.)
Scientific papers over the years have hypothesized that because male fetuses only have one X their disease would be so severe that they might not even develop to full term and the mothers might miscarry. There is no clinical data to support this hypothesis whatsoever.
Due to the sheer volume of sperm that is continuously made it is likely that all men produce sperm with MECP2 mutations. One in about 20,000 eggs will be fertilized with a sperm that has an MECP2 mutation in it – the cruel reality of genetic roulette.
by Monica Coenraads
There is no mystery about why a girl suffers from Rett Syndrome. The cause is the mutated copy of the MECP2 gene inhabiting her cells. But since MECP2 is on the X chromosome and all females have two X’s, beside each mutated gene rests a healthy but silenced twin. What if we could replace the flawed gene by reawakening its silenced counterpart? If we could wake up MECP2 in enough cells we could conceivably reverse Rett symptoms.

This is an approach that RSRT has championed since our launch in 2008. We are funding seven labs that are pursuing this line of work.
You may ask why do we need multiple labs working on the same goal. Isn’t that a waste of effort and money? The answer is a resounding “NO”. While the end game is the same each lab is using a different strategy to get there.
For example, the types of cells that labs are utilizing are different. Ben Philpot and colleagues at UNC are working with mouse neurons, Toni Bedalov and Jeannie Lee are using fibroblast cells, others still are using human cells. Each cell type has its own set of advantages and disadvantages.
The labs are also using different “reporters” – meaning how the cells are designed to detect activation of MECP2. Different compound libraries at different concentrations are being screened. Compounds are also being screened at various degrees of high and low throughput. And finally different criteria are being employed to define a “hit” (drugs that reactivate MECP2).

Bryan Roth gives us a tour of his robotic high-throughput
screening facility at UNC Chapel Hill
Having multiple labs attack this problem gives us more shots on goal and added assurances regarding the quality of any potential hits.
Two weeks ago we gathered everyone tackling this approach and brought them together for two intense days of talks and discussions.
Targeting MECP2 as a Treatment Strategy for Rett Syndrome
Chapel Hill, NC
May 12-13, 2014

Seventeen scientists from eight labs
plus advisors from NIH and industry participate at meeting in Chapel Hill, NC.
Over the past 15 years I’ve organized dozens of meetings and before each one I worry – will the discussions be forced or will they flow naturally? will collaborations ensue? It was no different with this meeting. The first few talks of the day however quickly put me at ease. While a number of common hits were reported in multiple labs much validating and further screening remains to be done. At the meeting, and in emails and phone calls since, the scientists are working out the logistics of validating each others hits, trading cell lines and compounds. Exactly the outcome I was hoping for.
Last year RSRT awarded a $750,000 grant to Michael Green, PhD of University of Massachusetts to pursue an unconventional approach to reversing Rett: reactivating the silent X chromosome. UMASS just released the piece and video below highlighting Dr. Green’s work. We are struck by the following quote from Dr. Green: “With NIH funding, you pretty much have to be doing mainstream research. The NIH doesn’t fund bold and innovative projects often. By contrast, organizations like RSRT are willing to take on high-risk projects that have controversial hypotheses and rationales, because these are the ones that really may have a great impact on disease.”
We thank all of our supporters who make it possible for us to fund innovative, out-of-the-box projects that we believe will move us towards a cure for Rett by leaps rather than small incremental steps.
From the UMASS Med NOW website:
UMMS scientist aiding a mother’s quest for rare disease cure
With a $750,000 grant from the Rett Syndrome Research Trust, Michael Green is working to reverse a debilitating neurological disease
By Lisa M. Larson and Bryan Goodchild (UMass Medical School Communications)
by Monica Coenraads
Variations in our genome are what make us unique. It’s also what predisposes or protects us from disease. For example, you may know people who eat high fat diets and yet have low cholesterol or people who, although they have never smoked, succumb to lung cancer, like Christopher Reeve’s wife, Dana.
I’ve had the opportunity to meet girls with MECP2 mutations and normal X chromosome inactivation that are too high functioning to be diagnosed clinically with Rett Syndrome. These are girls who may walk, run, speak, write, draw, and in some rare instances even speak multiple languages and play an instrument. So what is protecting these individuals from having full-blown Rett? You guessed it: modifier genes.
Those of you familiar with RSRT’s efforts know that we have been funding a project in the lab of Monica Justice aimed at identifying protective modifiers in mice. This past summer the Justice lab published the first modifier that suggests that statins (drugs that lower cholesterol) may be treatment options for Rett. More modifiers are likely to follow.
In the last few years a number of factors have coalesced to make the hunt for modifiers possible in people: 1) the identification of a growing number of individuals with MECP2 mutations who are too high functioning to fit the criteria for a clinical diagnosis of Rett 2) dropping costs for exome sequencing 3) improved bioinformatics which allow for better analysis and interpretation of the vast quantify of data generated from sequencing.
In light of these developments RSRT has awarded $314,000 to Jeffrey Neul at Baylor College of Medicine to sequence the exomes (the protein producing portion of the genome) of high-functioning kids/adults in the hopes that some common variables may point to modifiers which can then become drug targets.
Importantly, the sequencing and phenotypic data will be a valuable resource as it will be deposited into the National Database for Autism Research and available to the scientific community.
We need the Rett community’s help to identify high-functioning individuals who Dr. Neul may not be aware of.
If you think your child may qualify please contact me at monica@rsrt.org
Watch the interview below with Dr. Neul to learn more about this project.
by Monica Coenraads
[Italian translation]
[Spanish translation]
[Press Release]
Faced with the complex problem of discovering the elusive function of the Rett protein, RSRT set out to conduct an experiment of our own. We shook the conventional practice of laboratories working in isolation and instead convened three scientists to work collaboratively: the MECP2 Consortium. We gave them the necessary financial resources and provided infrastructure including in-person meetings. The results surprised us all.

Adrian Bird

Michael Greenberg

Gail Mandel
The MECP2 Consortium was launched in 2011 with a $1 million lead gift by Tony and Kathy Schoener.
RSRT has committed an additional $3.4 million of funding to the Consortium.
We are extremely grateful to the Schoeners for their second $1 million pledge to support this effort.
The Consortium quickly reported significant advancements. The Mandel and Bird labs showed, for the first time, a dramatic reversal of symptoms in fully symptomatic Rett mice using gene therapy techniques that could be utilized in people.
The “Rett mouse” moving around received healthy Mecp2 via gene therapy. The immobile mouse did not receive treatment. The video was taken four weeks after treatment.
The Bird lab discovered that the function of the Rett protein, MeCP2, depends on its ability to recruit a novel binding partner, NCoR/SMRT to DNA. Disrupt that ability and the symptoms of Rett ensue.
The Greenberg lab built on the work of the Bird lab and discovered that adding a phosphate group to MeCP2 alters its ability to interact with NCoR/SMRT and affects the expression of downstream genes.
While the clinical implications of the gene therapy experiments are obvious some may think “so what?” when it comes to the NCoR experiments.
I suspect that in the mind of many Rett parents the best evidence of research progress is clinical trials. However, this is often not the best measure of progress.
Thomas Südhof, recent Nobel Laureate, recently commented “I strongly feel that attempts to bypass a basic understanding of disease and just to get to therapies immediately are a misguided and extremely expensive mistake. The fact is that for many of the diseases we are working on, we just don’t have an understanding at all of the pathogenesis. There really is not much to translate. So NIH and many disease foundations are pouring money into clinical trials based on the most feeble hypotheses.”
So I will argue that investing in a better understanding of MECP2 – a primary goal of this Consortium – is money well spent, as it will add to our current arsenal of strategic approaches to combat Rett.
A repurposed drug may partially treat some of the symptoms, but to achieve the kind of dramatic improvement that most parents and I ache for will likely require attacking the problem at its very root.
As Rett parents will attest to the symptoms of the disorder are numerous and devastating. Whatever MECP2 is doing, it’s acting globally on many systems in the body. A repurposed drug may partially treat some of the symptoms but to achieve the kind of dramatic improvement that most parents and I ache for will likely require attacking the problem at its very root.
There are multiple ways to achieve this end goal: gene and/or protein therapy, activating the silent MECP2, modifier genes. These are all areas in which RSRT is financially and intellectually engaged with.
In parallel, however, it is imperative to understand what MECP2 does. RSRT has therefore committed an additional $3.4 million of funding to the MECP2 Consortium. We are extremely grateful to Tony and Kathy Schoener for their second $1 million pledge to support this important project.
I recently discussed the experiences of the past few years and what lies ahead with the Consortium members.
Greenberg: Research in neuroscience is undergoing a revolution. We now have the technologies in hand to solve some of the most difficult neurobiological questions. However, progress towards answering these hard questions requires scientists working together. A single lab working alone doesn’t have the expertise or the resources to make significant progress when the scientific problem is particularly challenging.
The MECP2 Consortium is a model for something much bigger: how neuroscience overall needs to operate so that we can find therapies and cures for disease.
The MECP2 Consortium is a model for something much bigger: how neuroscience overall needs to operate so that we can find therapies and cures for disease. We are scientists in different parts of the world, working together, sharing their results long before publication, and brainstorming openly on a regular basis. The different perspectives of the three labs allow for a wonderful exchange of ideas to advance the science. I believe this is what the Consortium is all about. We have ignored the typical barriers of geography and have brought together scientists from Edinburgh, Portland, and Boston on a regular basis. The results have been stunning. There has been much more rapid progress than would have been made by the individual labs.

Consortium meeting in Boston in November of 2013.
Bird: I agree. An over arching goal of the Consortium is to understand the way the MECP2 protein works at the molecular level. We are at last starting to make real progress on this and will be testing some of the new ideas in cellular and animal models. Our ultimate aim is to use this new knowledge to provide rational approaches to therapy.
Mandel: Front and center is always our goal to find a therapy for Rett. This guides our experiments and keeps us focused. The fact that financial support comes from families who have a child with Rett and their networks makes us work harder.
Coenraads: In your opinion what are the elements that have made this consortium “work”?
Greenberg: Trust and openness, a willingness on the part of all three Principal Investigators to talk through any potential problems immediately as they come up. A willingness to check egos at the door so that we can work together for something that is more important than our individual advancement. Importantly the participants, Mandel, Bird, Greenberg and Coenraads like and trust each other.
 Bird: We all have different backgrounds and interests, but we share a commitment to understanding Rett Syndrome. We compliment each other surprisingly well.
Bird: We all have different backgrounds and interests, but we share a commitment to understanding Rett Syndrome. We compliment each other surprisingly well.
Mandel: The regular meetings and exchanges and the quality of the scientists involved have been key factors as well as the availability of sufficient funding for each of us to follow our scientific noses.
Coenraads: Fortunately science is not linear. There are technologies available now that weren’t available when the Consortium started. How does this impact your Rett research?
Greenberg: There are a lot of new technologies available – in particular Cre lines that will allow us to study the effect of MeCP2 loss in a relatively homogeneous population of neurons, CRISPR and Talen technology that will facilitate gene correction, and genomic technologies that are providing a new understanding of the role of methylation in the control of neuronal gene expression. Also, better equipment, such as microscopy will help.
Bird: The technologies for genetic modification have existed for a decade, but the advent of CRISPR has made this facile. Being able to edit genetic mistakes in patients is no longer a science fiction dream, but has become a real possibility. Exploring this option will be an important focus for the Consortium.
Coenraads: Harrison Gabel from Mike’s lab recently shared with me in an email: Our group meetings are essential to critically assessing our work. Each lab group has its own “world view,” and having that view shaken up every six months is very constructive.
So I look forward to lots more critical assessments and worldviews getting shaken as together we get to the bottom of what MeCP2 does.
* Due to the success of the MECP2 Consortium, and its positive gene therapy findings, RSRT has just announced funding for a second consortium: the MECP2 Gene Therapy Consortium. Read more about this newly formed second collaboration.
by Monica Coenraads
[Italian translation]
[Spanish translation]
[Press release]
The videos below are perhaps the most well-known in the Rett community. If you love a child with Rett then chances are you’ve watched them obsessively.
This work published in 2007 by Adrian Bird, declared to the world that Rett is reversible, but did not tell us how this could be done in people.
Fast-forward six years and the video below from the RSRT-funded labs of Gail Mandel and Adrian Bird may have given us an answer: gene therapy.
The mouse moving around was given gene therapy treatment and received healthy Mecp2 gene. The immobile mouse did not receive treatment. The video was taken four weeks after treatment.
So how do we make the giant leap from recovered mice to recovered children?
To move us towards this goal, RSRT has launched their second collaborative group – the MECP2 Gene Therapy Consortium. This new group comes after the success of RSRT’s MECP2 Consortium, established in 2011, that led to the initial encouraging gene therapy findings. With a budget of $1.5 million the members of this international gene therapy collaboration are charged with tackling the necessary experiments to get us to clinical trials as quickly as possible.
I recently caught up with the investigators to discuss this novel collaboration:
Brian Kaspar (Nationwide Children’s Hospital)
Currently working on gene therapy clinical trial for Spinal Muscular Atrophy
Stuart Cobb (University of Glasgow)
Neurophysiology lab and co-author on 2007 reversal paper with Adrian Bird
Steven Gray (UNC Chapel Hill)
Currently working on gene therapy clinical trial for Giant Axonal Neuropathy
Gail Mandel (OHSU)
Member of MECP2 Consortium and author of gene therapy paper published this summer

Brain Kaspar

Stuart Cobb

Steven Gray

Gail Mandel
Coenraads: Let’s jump right in. Why Rett? Why now?
Cobb: While there have been major advances in understanding the molecular actions of the MeCP2 protein, it is still difficult to conceive of a small ‘traditional’ drug molecule being able to mimic its function. While traditional drug approaches will likely be restricted to correcting specific aspects of what goes wrong in Rett it is conceivable that gene therapy can correct the cause of Rett at its very source and thus provide a profound recovery of function.
While traditional drug approaches will likely be restricted to correcting specific aspects of what goes wrong in Rett it is conceivable that gene therapy can correct the cause of Rett at its very source and thus provide a profound recovery of function. – Stuart Cobb
Mandel: It has been known for some time now that when MeCP2 is expressed genetically in cells throughout an MeCP2-deficient mouse, major Rett symptoms are reversible in mice. Two of the big outstanding questions then are: 1) Will this be true for humans and 2) Can we add MeCP2 back to patients and also achieve reversal? The first question is currently still an open question, although upcoming experiments using human neurons and astrocytes derived from iPSCs and xenografts (transplanting human cells into mice) may provide some important clues. The second question is challenging because currently there are no reliable ways to introduce MeCP2 throughout the brain, although recent studies in mice, funded through RSRT consortiums, suggest that AAV9-mediated transduction (delivery via a virus) might have potential. Therefore, two advancing technologies, iPSCs and AAV9 viruses, are converging to compel us to jump right in now.
Kaspar: A major advantage in Rett is that the genetic target is defined for us: MeCP2. Another advantage is that it’s not neurodegenerative – neurons don’t die. And importantly, we know that restoring the proper level of MeCP2, even later in life, at least in a mouse, results in dramatic improvements.
Why now? Because the gene therapy field now has an arsenal of powerful new tools. We have at our disposal a tool kit that can express genes for long periods of time and that can target many cell types efficiently throughout the entire nervous system. Our challenge will be to utilize our toolkit to hit the precise cells at the right expression levels. I’m certain we can accomplish this goal.
Gray: That said, the devil is in the details. We have to get MeCP2 broadly distributed throughout the whole brain, which is something that has been done in animals but not yet in humans. Just as important, we have to be very careful to get the level of MeCP2 correct – too little may not work well enough and too much could cause a different spectrum of disease.
Coenraads: What have we learned thus far regarding gene therapy for Rett?
We’ve learned that a single one-time administration of a gene therapeutic can have a clinically meaningful result in the workhorse rodent model of this disease, even when delivered later in life. The results have been quite promising, and now multiple laboratories have similar promising results, it’s not just an isolated manuscript happening in one laboratory. – Brian Kaspar
Kaspar: We’ve learned that a single one-time administration of a gene therapeutic can have a clinically meaningful result in the workhorse rodent model of this disease, even when delivered later in life. The results have been quite promising, and now multiple laboratories have similar promising results, it’s not just an isolated manuscript happening in one laboratory. Using similar approaches, multiple groups have encouraging results. That’s good for science and that’s good for Rett patients.

Steve Gray (far right) and Stuart Cobb (second from the right) at an RSRT science meeting in late 2012.
Cobb: The studies have also shown that the level of MeCP2 protein produced by the gene therapy is not producing any obvious defects in its own right and it therefore seems possible to deliver protein within limits that are tolerable to cells.
We have also learned that it is not necessary to ‘hit’ all cells with the virus, this is never going to be achievable in practice anyway. Fortunately, a substantial therapeutic impact may be achieved by delivering the gene to a subset of cells. Of course the absolute number of cells, the types of cells and location in the brain is likely to be very significant. These are important issues that will be investigated by the MECP2 Gene Therapy Consortium.
Gray: Finally, the studies tell us that we have to be very careful how we target the MeCP2 gene, to make sure too much isn’t delivered to a particular organ, such as the liver.
Coenraads: Have you ever worked in collaboration with multiple labs? What do you think are the advantages? Could there be disadvantages?
Mandel: I have been fortunate enough to be part of a productive collaboration funded by RSRT to work on how MeCP2 functions normally, and in mutants, and to do, with Kaspar’s group and Adrian Bird, the initial pilot proof of principle for gene therapy for Rett, using AAV9 vectors.
Gray: Most of my work is done in collaboration with other labs, and I’m very comfortable doing research that way. I have a small and fairly specialized lab. We aren’t experts at everything, and it is much more efficient to collaborate with someone that has expertise than try to develop it on your own. This speeds things up and raises the quality of the work. The keys to making it work are that everyone has to be fully committed, and there has to be a level of trust across the members of the consortium. Trust that you can share data openly, and trust that the work is being carried out to the highest standards. If one investigator isn’t doing their part or does sloppy science then things can fall apart.

Stuart Cobb (right) with David Katz.
Cobb: I have enjoyed a number of successful bilateral collaborations in the past but the formation of the four-lab Consortium is going to be a new venture for me. Clearly there will be big advantages in terms of pooling complementary expertise to make swift progress. However, there will also be challenges, one being the necessity to maintain very good communication within the Consortium to coordinate our efforts and work together efficiently.
Kaspar: My laboratory is engaged in a number of collaborations and they are a major reason we have been successful. Our international collaborations have given us access to patient samples as well as opened the door to new ideas and interactions that just couldn’t be accomplished sitting in isolation. Collaborations bring everyone’s experience and expertise to the table and allow the participants to rapidly answer difficult questions. We don’t always have to reach consensus but the right team will be open to sharing ideas and comfortable with hearing criticism as well as be aligned on goals and focused on the patients.
Coenraads: What are the strengths your lab brings to the table?
-

Steve Gray in his lab at UNC Chapel Hill
Gray: We are part of one of the best gene therapy centers in the world, with a vector core facility that makes hundreds of research preps and several clinical preps each year. My lab in particular has, as its primary goal, a mission to develop nervous system gene therapy platforms. We’ve made enormous strides using existing vectors to their full potential, and also leading the way to develop newer and better vectors. Also, our experience bringing our Giant Axonal Neuropathy project to clinical trial gave us experience on the process of moving a biological from the bench to the bedside.
Cobb: My own lab brings expertise in the neurobiology side in terms of accurately mapping out Rett syndrome-like features in mice and within the brain and being able to assess in detail the ability for gene therapy to improve aspects of the disorder.
Mandel: I am a basic science lab and I have strengths in applying state of the art molecular tools to questions related to gene therapy. My lab also has much expertise in histology of the brain.
Kaspar: We have successfully navigated two programs from bench research to human clinical trials. We have flexibility to focus on complex basic biology questions, while keeping in mind our goal to advance therapies towards human clinical trials.
Coenraads: Gene therapy has had a rocky road. How do you view the field at the moment?
Kaspar: Expectations and promises were far too high in the early days of gene therapy. I think transformative therapies go through this track of failing and then triumphing. One simply has to look at the field of organ transplantation as an example. I think gene therapy will triumph, but we still have much to learn and pay attention to. There is a great deal of excitement and hope in the field today. We have to be good custodians of this technology with laser focus on safety and design of human clinical trials.
Gray: There are a lot of good things happening in the field right now with patients seeing major improvements in their lives as a result of gene therapy. The first gene therapy product received full regulatory approval last year in Europe. Biotechnology companies are taking an interest in gene therapy. Frankly, it is a good time to be in the field.
Modern, safer, approaches to gene therapy are developing very rapidly and it is one of the most vibrant fields in the genetics and molecular medicine arena at the moment. – Stuart Cobb
Mandel: I think that there is a large and growing momentum now for gene therapy because of the huge advances in molecular biology and viral technologies.
Coenraads: I find that the gene therapy area is polarizing – people love it or hate it – have you encountered a similar response?
Cobb: Yes, I have indeed encountered such contrasting views. Even within the community of Rett clinicians, I have had views of gene therapy being ‘the obvious route to follow’ versus others expressing great skepticism. Interestingly, the view within industry has been more accepting, perhaps due to the massive shift towards biologicals (alternatives to classical small molecule drugs) that has occurred in recent years.
Kaspar: Typically those that are not fans of this technology focus on past failures. With any transformative findings there will be disbelievers. I’m reminded by a quote from Alexander von Humboldt: There are three stages of scientific discovery: first people deny it is true; then they deny it is important; finally they credit the wrong person.
Gray: I can’t blame some people for hating it. Gene therapy promised a lot early on, before the technology was very developed. Expectations should have been tempered somewhat while the science was worked out, but instead the field moved too fast and people got hurt. That said, I don’t think you should turn your back on a potentially revolutionary medical technology because of mistakes made over a decade ago when the field was in its infancy. If you take a fresh look at the things happening today, there is a lot of real and well-founded optimism.

Gail Mandel at a
recent RSRT meeting.
Mandel: As in any area of science, there are proponents and detractors. There are technical issues with gene therapy, such as scaling and side effects that need to be addressed before more people will lose some skepticism, although some skepticism is quite healthy and pushes us to be as rigorous as possible.
Coenraads: Dr. Kaspar, tell us a bit about your experience bringing the Spinal Muscular Atrophy project to clinical trial. How long did it take from mouse experiments to trial? How much money was invested from your lab?

Brian Kaspar (middle) at an RSRT workshop.
Kaspar: Our SMA program is quite exciting. We discovered the unique capacity for AAV9 to cross the blood brain barrier in 2009, in 2010 we were in progress to have the longest living SMA mouse in the world. We further tested safety and navigated the regulatory process including the NIH Recombinant Advisory Committee, the Food and Drug Administration and our institutional review board. Late in 2013 we were granted approval from the FDA and we will be injecting our first patients in a Phase 1/2 clinical trial early this year. It was a hectic 3-year process that cost $4 million and counting. We are excited and hopeful to help children with SMA type 1.
Coenraads: Dr. Gray, you are developing a gene therapy treatment for a disease called Giant Axonal Neuropathy. Can you tell us about your experience with that project? How far from clinical trials are you? How long did it take from mouse experiments to trial? How much money did it cost?
Gray: My GAN project has been life changing. This was the project that made the connection for me to patients and changed the way I think about research. Before then it was just about getting a good paper, or a grant, or doing the right things to advance my career. Now it is about making a real difference in the lives of people I’ve come to know and love. We’re on track to treat the first patient in the first half of 2014. We developed the treatment about 3 ½ years after starting the project, which included testing the treatment in the laboratory and developing an approach that should translate to humans. It’s taken another two years to start the trial. Our preclinical supporting studies were approximately $1.5 million. The FDA-required safety studies were another $0.75 million. We are budgeting another $1.5 million for the clinical trial. Most of these funds were provided by a small grass-roots foundation called Hannah’s Hope Fund.
Coenraads: I’m delighted that you have all agreed to collaborate. I look forward to our bi-monthly phone calls and in-person meetings twice a year. Parents all over the world will be waiting anxiously to hear about your progress. As you know, there is a lot at stake.
by Katie Bowers and Monica Coenraads
Sometimes, new ideas come from the strangest of places; inspired by something that seems completely unrelated. This sort of out-of-the-blue brain blast is exactly what happened in 2012 when a study about bacteria’s adaptive immunity opened up the possibility for a new approach to gene therapy. While CRISPR technology was runner-up for the Breakthrough Technology of the Year in 2012, its presence in bacteria cells has been known since 1987. It was not until a series of studies between 2005 and 2007, however, that their role in the bacterial adaptive immunity was revealed.
Short for Clustered Regularly Interspaced Short Palindromic Repeats; a CRISPR is a small length of DNA with a repeating and reversing sequence of base pairs (the pieces that make up genetic code). Found in a specific section (or loci) in the bacterial genome, multiple CRISPRs are usually grouped together in a string. Each CRISPR is followed by a ‘spacer’ section of DNA.
When pathogens, such as a virus, attack bacteria they insert a section of DNA into their victim. Oblivious to any danger the victim reads and processes this foreign DNA. In so doing, the bacteria also retain a record within its spacers enabling it to recognize and fight off the same pathogen in the future. Each spacer therefore acts as a ‘bookmark’ for any pathogen attack the cell has encountered.
How do bacteria do this? Preceding a CRISPR sequence are cas genes which make enzymes that copy the foreign DNA sequence and insert it as small fragments into the bacterial genome as new spacers.
With this new spacer now available in the CRISPR loci, the next time a bacteria encounters the same attack it will recognize the foreign DNA and send out a secondary Cas protein to target, bind, and splice out the DNA thereby inactivating the invader, analogous to the way in which our bodies produce antibodies against repeated infections
It’s this ability to identify, isolate, and splice out a specific piece of DNA that has captured the imagination of scientists.
The CRISPR/Cas system can now be hijacked to accurately target genetic errors in DNA and remove them. Once removed repair systems kick in to fix the sequence. This can be done either of two ways: non-homologous end-joining which simply connects the two ends together or the more sophisticated homology-directed repair.

Image: Courtesy of Broad Institute
Applying this to Rett Syndrome we can use the following example. The most common mutation in MECP2 is the T158M where a cytosine to thymine error at nucleotide base number 473 swaps a methionine amino acid for a threonine. One could envision using the CRISPR/Cas technology to introduce a cut at nucleotide base number 473, splice out the thymine and provide a cytosine base via a template. Cas enzymes and base repair templates would be delivered via gene therapy vectors.
So where does the technology currently stand to be able to achieve this? Inducing the break in DNA can now be achieved efficiently and effectively however the repair step using templates is not yet ready for prime time as efficiency rates (the number of cells that actually achieve repair) are still quite small. Before the technology can be considered for therapeutic applications it also needs to be shown that the CRISPR/Cas machinery only cuts the DNA at desired sites as additional “off target” breaks could be damaging.
The encouraging news is that progress with CRISPR/Cas technology is occurring at lightning speed with thousands of laboratories around the world working to improve the process. It is not difficult to imagine the immense possibilities for treating genetic disease. No wonder there is so much excitement with scientists themselves routinely referring to this new technology as revolutionary.
We are starting the New Year with the wonderful news that Professor Adrian Bird has been Knighted for his services to science. For anyone following Rett research Prof. Bird needs no introduction. His list of contributions to the Rett field are numerous starting with the discovery of the MeCP2 protein in the early 1990’s to the development of the first animal model in 2001 to the unexpected discovery that Rett symptoms are reversible. We congratulate Sir Adrian Bird and wish him the best for 2014 – may the discoveries continue!

Professor Adrian Bird

Prof Bird with fellow trustees Heidi Epstein,
Monica Coenraads and Marci Valner

At the end of every year the Simons Foundation announces its list of most notable papers from the autism field. Among these are two RSRT-funded papers: the gene therapy paper from Gail Mandel and Adrian Bird and the statin paper from Monica Justice.
It seems that cholesterol and the brain is becoming a hot field. Following up on the RSRT-funded results of the Justice lab comes an intriguing (and unpublished) study highlighted by the Simons Foundation.
New autism gene plays key role in cholesterol synthesis
 Mutations in a gene that plays a role in producing cholesterol in the body increase the risk for autism, pointing the way toward therapies for some people with the disorder. The research was presented in a poster Tuesday at the Autism Consortium’s 2013 Research Symposium.
Mutations in a gene that plays a role in producing cholesterol in the body increase the risk for autism, pointing the way toward therapies for some people with the disorder. The research was presented in a poster Tuesday at the Autism Consortium’s 2013 Research Symposium.
Mutations in the gene, DHCR24, are known to result in a severe metabolic disease linked to cholesterol. The gene’s newly discovered role in autism suggests that statins, drugs that lower cholesterol levels, may treat symptoms of autism.
“The potential is exciting,” says Timothy Yu, a neurologist at Massachusetts General Hospital and a researcher at the Broad Institute of the Massachusetts Institute of Technology and Harvard University in Cambridge, Massachusetts.
“If you can find these kids who are swimming around in an otherwise generic autism pool and figure out a way to treat them appropriately, then you actually have the possibility of therapy.”
The researchers screened 2,000 families that have at least one child with autism to identify rare recessive gene variants for the disorder. In one family, they found that a girl diagnosed with pervasive developmental disorder-not otherwise specified and two boys diagnosed with intellectual disability and autism had all inherited two copies of the mutation, one from each parent.
“The gene is involved with the cholesterol synthesis pathway, so it started us thinking about the pathway’s role in autism and intellectual disability,” says Elaine Lim, a graduate student in Mark Daly’s lab at Harvard who presented the research.
To profoundly impact a disorder with as many varied and debilitating symptoms as Rett Syndrome, it is likely that intervention must be directed toward the very root of the problem. There are several ways to do this: activate the silent back-up copy of the Rett gene; target modifier genes; explore gene therapy.
Today, we announce a study funded through the MECP2 Consortium suggesting that gene therapy may indeed provide a feasible approach to treat Rett Syndrome.
The work was led by Gail Mandel at Oregon Health and Sciences University in collaboration with Adrian Bird of the University of Edinburgh and Brian Kaspar of Nationwide Children’s Hospital.
 Gail Mandel with lab members |
 Adrian Bird and post-doc |
In the past sixty days, four key papers have been published detailing research advances supported financially and intellectually by RSRT. Three of those papers are funded through the MECP2 Consortium, a unique alliance launched by RSRT in 2011 among three leading labs: Bird, Greenberg (Harvard) and Mandel. If you are a donor to RSRT, the accelerated research these projects represent is the result of your money at work.
We wish to express our gratitude to all of our generous supporters and the parent organizations that make this progress possible. Special thanks to our funding partners, the Rett Syndrome Research Trust UK and the Rett Syndrome Research & Treatment Foundation.
Below are some resources to help you understand today’s announcement.
Press Release [Spanish Translation] [German Translation]
Video interview with Dr. Mandel & lab members
In contrast to the leadership of most organizations we yearn for the day when RSRT is no longer in business – that will mean an end to Rett. Until that day comes we will continue to invest in high quality science. In the first half of 2013 RSRT has committed $1.7 million to new projects that range from basic science to clinical trials. We invite you to learn about our investments, which our donors have made possible. As always we welcome your questions and feedback.
Copaxone Clinical Trials
There is a multitude of data suggesting that mice models of Rett have low levels of a neurotrophic factor called BDNF (brain derived neurotrophic factor). BDNF is a very important and complex protein that is implicated in a variety of disorders. Increasing BDNF in the Rett mice models, either genetically or pharmacologically is beneficial. An FDA approved drug for multiple sclerosis called copaxone (or Glatiramer Acetate) is known for increasing BDNF and therefore of interest in treating Rett.
RSRT has committed to funding an open label study of copaxone in two centers, the Tri-State Rett Syndrome Center at Children’s Hospital at Montefiore in the Bronx, under the supervision of Dr. Sasha Djukic, and at Sheba Medical Center in Ramat Gan in Israel under the supervision of Dr. Bruria Ben Zeev. Each center will give copaxone to ten individuals for 6 months. Below is a comparison of the two studies.

Sasha Djukic, M.D., Ph.D.

Bruria Ben Zeev, M.D.
| USA | Israel | |
| Title | Pharmacological treatment of Rett Syndrome with Glatiramer Acetate (Copaxone) | An open-label exploratory study to investigate the treatment effect of glatiramer acetate (Copaxone) on girls with Rett Syndrome |
| Principal Investigator | Aleksandra Djukic, MD, PhD | Bruria Ben Zeev, MD |
| Location | Children’s Hospital at Montefiore, Bronx | Sheba Medical Center, Ramat Gan, Israel |
| Objectives | Primary: gaitSecondary: cognition, autonomic function, EEG, quality of life | Primary: EEG improvementSecondary: autonomic function, general behavior, communication, hand stereotyping, feeding, gastrointestinal |
| Study Size | 10 girls – 10 yrs old and up | 10 girls – 6 to 15 yrs old |
| Dose (injections) | Ramp up to 20 mg per day | Ramp up to 20 mg per day |
| Length of study | 6 months | 6 months |
While copaxone is not going to cure Rett Syndrome we hope that by increasing BDNF we will see improvements in symptoms. The trials are currently recruiting.
The X Factor
If you’ve been following RSRT’s activities then you know that one of the strategies we are pursuing is reactivation of the silent MECP2. We are adding Michael Green of UMass to our existing portfolio of labs who are working in this space.

Michael Green, M.D., Ph.D.
Dr. Green is taking a somewhat unconventional approach as he is attempting to reactivate the entire X chromosome and not just MECP2. His work first came to RSRT’s attention in 2009. We learned that he was conducting a screen to identify genes that control X chromosome inactivation (XCI). As his work matured over the next few years he did indeed identify factors that control XCI, some of which belong to molecular pathways for which there are drugs. These drugs can now be tested in culture and in vivo in the Rett mice models.
Recently a paper from a colleague of Michael Green’s, Jeanne Lawrence, received enormous attention for doing the opposite of what Dr. Green proposes – inactivating the extra copy of chromosome 21 that causes Downs Syndrome (DS). While this work is not ready for prime time it is an exciting new avenue that could eventually make treatment for DS a reality.
Continuing our X Factor focus we awarded an additional grant to Jeannie Lee of Harvard University. She is currently funded for a drug screen to reactivate MECP2. While the goal of the new award is the same – activate MECP2 – how she proposes to accomplish it is completely novel.

Jeannie Lee, Ph.D. at an
RSRT meeting in November
The hypothesis of Dr. Lee’s approach rests on an observation that a group of proteins called Polycomb complexes working in concert with a certain type of RNA, called noncoding RNA (lncRNA) are relevant for keeping genes silent on the inactive X.
Dr. Lee’s therapeutic strategy is to awaken the MECP2 gene by disrupting the binding that occurs between the lncRNA and the Polycomb complexes.
While the work is early stage, if Dr. Lee’s hypothesis proves correct this approach would be attractive.
Ketamine – the follow up
Last October David Katz of Case Western Reserve University published a paper showing that certain physiological symptoms in the Rett mice normalized after treatment with an aesthetic called ketamine. With RSRT funding Dr. Katz will continue to pursue this line of exploration with additional drugs that work in the same pathway but have less side effects. He will also attempt combination therapies with various drugs.
Below is a video interview with Dr. Katz that was posted earlier this year.

Kevin Foust (left) and Brian Kaspar
at a recent RSRT meeting.
The final award was granted to Kevin Foust of Ohio State University. Dr. Foust has been working with Brian Kaspar and Gail Mandel on gene therapy approaches to treat Rett Syndrome. This award builds on that work by extending a gene therapy approach to the MECP2 Duplication Syndrome. Dr. Foust will deliver RNA interference (RNAi), a biological process in which RNA inhibits protein production) via a vector in duplication syndrome mice.
If the data is encouraging this work would form the basis for a therapeutic approach to treating patients.
This work is being funded via the MECP2 Duplication Syndrome Fund at RSRT and directly supported through the efforts of the duplication families.
We look forward to bringing you updates on these and all of our projects. Once again we thank all of our supporters – this is your money at work for our girls.

Monica Justice, Ph.D.
Rett Syndrome is a spectrum disorder with a broad range of symptom severity. Some girls can run, have some use of their hands and can speak in short sentences while others cannot even sit or manage to hold their head up. One reason for this variation is the child’s own unique genetic make-up – in other words, variations in other genes that impact the severity of the Rett mutation. Identification of modifier genes has therefore been a critical component of RSRT’s research program as the modifiers may provide alternate pathways to target.
This hypothesis has now been supported in a major study that could lead to treatments for girls and women with Rett Syndrome. Today the journal Nature Genetics publishes data on the first reported modifier, called Sqle, an enzyme involved in the cholesterol pathway.

Monica Justice, Ph.D and graduate student Christie Buchovecky from Baylor College of Medicine.
The research was undertaken by Monica Justice, PhD, of Baylor College of Medicine, with a $1.5 million investment from RSRT. Dr. Justice tested statins (cholesterol-lowering drugs) on Rett mice models with encouraging results. A human clinical trial is now being planned.
RSRT is committed to seeing this project through to completion as many more modifiers, and therefore druggable pathways, are likely to be found. We thank all of our generous supporters and parent organizations who make this important work possible, in particular our funding partners, Rett Syndrome Research Trust UK and the Rett Syndrome Research & Treatment Foundation.

Below are some resources to help you understand today’s announcement.
Press Release Announces Tim’s Hire
Dear Friends,
 A year and a half ago my wife, Rachel, and I received the worst phone call of our lives—it was the Children’s Hospital of Pennsylvania informing us that our beautiful, bright-eyed, giggling two-and-a-half year old daughter, Eleanor, had tested positive for the MECP2 mutation that causes Rett Syndrome. We were simply devastated and didn’t know what to do or where to turn. The ensuing months were the hardest of our lives. Our dreams and hopes for our only child had been crushed. We watched helplessly as Eleanor stopped scooting on her rear end, as she had been doing, and developed a repetitive hand motion and other symptoms.
A year and a half ago my wife, Rachel, and I received the worst phone call of our lives—it was the Children’s Hospital of Pennsylvania informing us that our beautiful, bright-eyed, giggling two-and-a-half year old daughter, Eleanor, had tested positive for the MECP2 mutation that causes Rett Syndrome. We were simply devastated and didn’t know what to do or where to turn. The ensuing months were the hardest of our lives. Our dreams and hopes for our only child had been crushed. We watched helplessly as Eleanor stopped scooting on her rear end, as she had been doing, and developed a repetitive hand motion and other symptoms.
Several months later, Rachel found the RSRT website. We made a call and RSRT’s co-founder and executive director, Monica Coenraads, answered the phone. We soon learned that we were not alone in our sadness and that there was a community of wonderful, supportive parents, grandparents, family members, and friends of families who have been impacted by Rett Syndrome. And even more exciting, we learned that there was hope for a cure for Rett. In fact, it was clear that there was a good deal more than hope—Rett symptoms had been reversed in an animal model, and very promising scientific progress was being made, much of it encouraged and supported by RSRT.
Monica and I continued to correspond. I was seeking her advice and thoughts about Eleanor’s diagnosis, and I was trying to understand the research and science. Monica began seeking my advice about fundraising and public relations when she learned that I headed the development office of the Woodrow Wilson Foundation in Princeton, New Jersey, and before that had served as director of development at Columbia University’s Teachers College. It may have dawned on Monica and me at the same time that there was a fit here—that I cared deeply and personally about the work that RSRT was doing and that I had nearly 20 years of experience that could help grow RSRT and its impact on the lives of girls and women with Rett.
 The rest, to use the cliché, is history. My first official day as a Program Director at RSRT was June 17. Under Monica’s direction, I’m responsible for fundraising, public relations, and strategic thinking about the organization. I couldn’t be more excited. So many people, Monica foremost among them, have worked so hard and contributed so much to making RSRT the respected force that it is and to building the cumulative scientific knowledge that will lead to a cure. I’m honored and humbled to join this team. Frankly, I never imagined that I would be able to put my knowledge and skills to use for something so important to me.
The rest, to use the cliché, is history. My first official day as a Program Director at RSRT was June 17. Under Monica’s direction, I’m responsible for fundraising, public relations, and strategic thinking about the organization. I couldn’t be more excited. So many people, Monica foremost among them, have worked so hard and contributed so much to making RSRT the respected force that it is and to building the cumulative scientific knowledge that will lead to a cure. I’m honored and humbled to join this team. Frankly, I never imagined that I would be able to put my knowledge and skills to use for something so important to me.
I’ve gone on longer than I intended, but I have one further thought. I’ve been thinking lately about President Kennedy’s 1961 speech to Congress in which he announce the dramatic and ambitious goal of sending an American to the Moon before the end of the decade. It took a huge team effort, conviction and confidence in a very clear mission, ample resources, and leadership, for the goal to be met. I think RSRT’s goal is not unlike President Kennedy’s in its ambition, its clarity, its importance, and its attainability. I also believe that all of us working together are the team that will get us to the moon. And, like building a rocket, a cure depends on cumulative knowledge that is the sum of its parts. The rocket needs its nose cone, its fuel tank, its electronics, its landing pads, and other components to meet the goal. Research is like this too. All the parts have to work together. It is cumulative knowledge that will get us there.
 I am tremendously grateful to Monica, to the RSRT Board, and to all of you who contribute your time, energy, and resources to RSRT for your confidence in me. I promise Eleanor and all of our daughters that I will do my best in everything I do for RSRT. I will need your help, advice, and counsel—most of you know RSRT and all of its accomplishments and the Rett community at large far better than I do—so I hope I can call on you. Please don’t hesitate to contact me any time. My office line is 609-309-5676; my cell is 609-815-5102; and my email is tim@rsrt.org. I look forward to meeting you.
I am tremendously grateful to Monica, to the RSRT Board, and to all of you who contribute your time, energy, and resources to RSRT for your confidence in me. I promise Eleanor and all of our daughters that I will do my best in everything I do for RSRT. I will need your help, advice, and counsel—most of you know RSRT and all of its accomplishments and the Rett community at large far better than I do—so I hope I can call on you. Please don’t hesitate to contact me any time. My office line is 609-309-5676; my cell is 609-815-5102; and my email is tim@rsrt.org. I look forward to meeting you.
Best,
Tim Freeman

Adrian Bird (left) and Matt Lyst
University of Edinburgh

Michael Greenberg (right) and Dan Ebert
Harvard Medical School
It stands to reason that in our battle to cure Rett Syndrome it would be of great benefit to understand the function of the “Rett protein”, MeCP2. Towards this end RSRT launched the MECP2 Consortium in 2011, a unique $1.8 MM collaboration between three distinguished scientists, Adrian Bird, Michael Greenberg, Gail Mandel. On June 16th the first two publications from this collaborative effort are published in Nature Neuroscience and Nature. Together these papers provide further clarification of the elusive function of the MeCP2 protein and how mutations within it contribute to Rett.
We thank Kathy and Tony Schoener whose visionary $1 MM gift made the Consortium possible. We thank all of our donors and parent organizations worldwide who support us, in particular our funding partners Rett Syndrome Research Trust UK and the Rett Syndrome Research & Treatment Foundation.
We are providing a variety of resources to help you understand the progress being reported today.
Animation of Nature Neuroscience Paper (courtesy of Jeff Canavan)
Chinese Translation
Interview with Matt Lyst, post-doc in Bird lab
Interview with Michael Greenberg and Dan Ebert,
post-doc in Greenberg lab
We break down a few interesting results recently published.
Multiple mutations on MeCP2
In most cases of Rett syndrome, one mutation occurs on (a single copy of) the MeCP2 gene. But, rarely, multiple mutations occur on the same copy. Researchers from the University of Alabama at Birmingham characterized the largest group of individuals to date, 15 of them, who have more than one mutation. (The girls were participating in the Rett Syndrome Natural History Study, which aims to gather detailed historical and physical examination data on a large cohort of females with Rett — of which there are now more than 800.)
In contrast to two previous case studies suggesting that girls with more than one MeCP2 tend to have more severe disease, the new study found that participants with multiple mutations assessed using two quantitative measures — the Clinical Severity Scale and the motor behavioral analysis — showed no difference from those individuals with single, similar mutations. The findings appear in the American Journal of Medical Genetics Part A.
Other factors, including the individual mutations themselves or how much of the mutated gene is inactivated as part of normal development, can worsen symptoms, the authors suggest. Future drug therapies that account for a person’s genetics should consider this small group of individuals, the authors add.
Rett Syndrome and epilepsy
Seizures affect 50% to 90% of individuals with Rett syndrome. According to a recent review in Pediatric Neurology by Alison Dolce and her colleagues at Johns Hopkins Hospital in Baltimore, Maryland:
- It’s unclear whether earlier onset of seizures signals a poorer overall prognosis.
- EEG, or electroencephalography, an indirect measure of brain activity, is an important diagnostic tool in children with Rett syndrome because it allows clinicians to identify true seizures — as opposed to symptoms, ranging from hyperventilation or motor abnormalities, that are often mistaken for seizures. From 2 to 4 years of age and beyond, all girls with Rett syndrome develop abnormal EEGs, the authors write.
- There are few studies assessing anticonvulsant therapies in Rett, and many of them are short-term and involve only a small number of participants. “At our centers, we have circumstantial evidence of good responses to levetiracetam, lamotrigine, and topiramate,” the authors write. Although the clinicians also use valproate, the side effects can be problematic. In general, many children with Rett require more than one medicine.
- It’s still difficult to predict which patients will tend to develop seizures early and also whose seizures will be easier to treat.
- According to the authors, physicians should begin to consider the ketogenic diet and vagal nerve stimulation earlier in the clinical course of Rett, because these treatments “might be helpful.”
More on MeCP2 mechanisms
MeCP2 protein has several defined parts that researchers are studying. The methyl-CpG-binding domain or MBD, for example, binds to DNA in studies conducted in vitro and is thought of as crucial for the protein’s function of turning genes on or off, or altering the way DNA is stored in a cell. Given that the interactions between MeCP2 and DNA are difficult to mimic in a dish, a team of Canadian scientists wanted to see what would happen to the interaction in a mouse whose front end of the protein was deleted. The Mecp2tm1.1Jae model, commonly used in studies of Rett syndrome, is missing its first 116 amino acids—which include the 48 amino acids that make up the MBD. (What’s more, MeCP2 mutations in people with Rett occur in this region, such as R106W, R1333C, P152R, F155S and T158M.)
In the study, mutant mice still had MeCP2 in their tissues but only about half as much as healthy mice did. Although the mutated MeCP2 still bound to DNA and chromatin, the interactions were weaker and less specific for methylated DNA. In addition, although the ‘nuclear localization signal’ region of the mutated protein was still intact, protein was less able to move from the cytoplasm to the nucleus, where it can influence the production of other proteins. The paper is published in the May issue of Nucleic Acids Research.

Guest blogger, Beth Johnsson, with her daughter Hannah.
by Beth Johnsson
Someone once told me that hope is what distinguishes humans from every other species; our ability to look to a potential future rather than live solely in the here and now. This is, of course, wildly inaccurate; I am not a biologist or anthropologist (or any other ‘ist’ for that matter), but I believe there are a number of other factors which separate humans from the species around us (not least, an opposable digit!). But the concept has stuck with me and, since most of my life is built around hope, it cannot help but strike a chord.
Three weeks ago, Hannah had four medical appointments in five days and a multitude of other issues which needed addressing. Hope permeated them all. I hoped, for example, that we would manage to get a disabled parking space at the hospital; I hoped that the wait wouldn’t be so long that we lost Hannah’s good will before we even got in the room; I hoped that the ECG results would show that the recent episodes we have observed are not seizures; I hoped that the locum SLT (who barely knows Hannah) would recognize that eye gaze is her best possible chance at communication and would therefore support our application for the technology; I hoped that the engineer would say he could adapt Hannah’s trike to make it big enough for a child who should be riding a bike; I hoped that the physio would approve a height adjustable bed to prevent our inevitable back injuries; I hoped the eye unit would finally discharge us because, (take note SLT!), her eyes do work; I hoped the blood tests results would require no more than three adults to hold Hannah down; most of all I hoped we would make it through each appointment without a total meltdown (Hannah’s, not mine!)
Sorry, that’s wrong, that is not what I hoped most of all. Most of all, I hoped, with every second of every appointment and of every minute in between, that one day none of this will be needed. I hoped that one day the ‘professionals’ involved in my little girl’s life will be her teachers, her GP and her dentist. And that’s all.
Two weeks ago, the routine medical appointments were replaced by an unexpected admission to hospital. Her first. (What a strange world we now live in, where I know that making it through six years without a hospital admission makes us incredibly lucky). An infection, the source of which remains unknown, has made my cheeky and (hitherto) still mobile little girl, lethargic, disengaged, and unwilling to walk. Most worryingly, it has put out the sparkle in her eyes. Now the professionals involved reach an all-time high, as does my reliance on hope. I watch her suddenly unable to take a step on her own, trembling violently, and I cling fiercely, with muscles I didn’t even know I have, to the hope that this is not the beginning of regression, the start of a life time spent in hospital, but ‘just’ the temporary result of an infection. Something they do have a cure for.
When Hannah was first diagnosed I didn’t know enough (and didn’t allow myself to know enough) about Rett Syndrome to understand that a cure was the only hope. Then, once I started to learn more, I didn’t allow myself to believe that a cure in her lifetime was possible. It seemed too fantastical, too far out of reach, to think it could really happen in time for my little girl. Now, as I continue to learn more about what Rett really means for Hannah, and about the research going on today, I realise that a cure is not only possible, it is the ONLY possible future. I cannot allow myself to think about the possibilities of the alternatives.
Nor can I help but be frustrated and confused by those who don’t seem to share or, at the very least, support that hope. Why do ‘friends’ click ‘like’ on every trivial Facebook message out there, but ignore my posts about Rett? Why can they not take the time to vote when funding is at stake? Why can they not spare the cost of a skinny latte to help make the hope reality? Why do they so often seem to think that I should just accept how things are? Would they? I hope not! It seems to me that to accept is to admit defeat; to hope is to fight. If my hope was false, my fight for an impossible prize, I could understand why acceptance might be healthier, more practical, but it is not false. The prayer I offer every night is not one born of blind faith, for a miraculous thunderbolt from an omniscient being; it is one born of proven fact, for a miraculous breakthrough by a handful of knowledgeable scientists, supported by a group of dedicated parents, in whom my faith lies and on whom my hope depends.
Perhaps others think I should accept because they wonder about the merits of a life based on hope, on a dream for the future. Carpe diem and all that? To be honest, sometimes I have wondered too. Shouldn’t we be living for today, enjoying what is here and now rather than always looking to a future which, ultimately, we cannot guarantee will arrive in time? But the two are not mutually exclusive, surely? In fact, I would say they are co-dependent. Living in hope for the future makes today more positive too: it enables you to notice the tiny, almost imperceptible steps being made forwards, the achievements which others might miss, but which you know are all part of the journey. Why should hoping for a brighter tomorrow preclude you from seeing the light in today? I don’t think it does; the light which research has switched on for Hannah’s future shines in her today too. It illuminates all the other reasons to be hopeful and grateful. When you are in darkness, finding the light switch is hard, so the darkness continues, self-perpetuates, the exit remains elusive. But when you have a little light shining already, no matter how small, finding your way towards the brighter, bigger light in the distance becomes an easier journey.
Before we discovered Rett Syndrome Research Trust and the research they fund, the sense of helplessness was overwhelming. For a control freak, like me, it was impossible. Uncertainty about the future is bad enough, but feeling there is nothing you can do to change it is torture. Even fundraising didn’t feel truly hopeful, when ultimately we knew money was going towards coping with diagnosis, not in making that diagnosis a demon of the past. When I look back on that time, I remember a very dark place, not simply because of the diagnosis itself, but also because of our lack of vision of the future or of how we could influence it. Hopelessness for the future meant helplessness today. The relief that comes from feeling that you are actually doing something, that you are taking action, raising money, helping to fund the science which is holding all your hopes in its hands, this relief is a light switch. Since we turned it on, both tomorrow and today have seemed a great deal brighter.
I started writing this, and thinking about hope, several weeks ago. Every time I think it’s done, something else comes along which is so tightly bound up with hope, some new experience or emotion which makes our hopes shift and metamorphose once more, that I have to start again. When I started writing, Hannah had never been in hospital overnight. She was walking confidently, progressing, even. She’d taken an independent step or two up the stairs. We were daring to hope she might continue. Now things have changed and with them, our hopes. Today we are hoping that she returns to where she was three weeks ago, now just that would be a little miracle. I’m sure all parents’ hopes for their children change over time, evolving inevitably as the child grows and develops their own set of hopes and dreams. I expected that. I just never thought that one morning I would wake up hoping that my six year old will bear her own weight. Everything is relative – our daily, weekly, monthly hopes change, but the ultimate hope is a constant, one of the few things in my daughter’s life which will not be lost.
I started, all those weeks ago, by talking about what distinguishes humans from other species. Speech, surely has to be one of our greatest gifts. The very thing I hope for most for my daughter. I make a joke of the opposable digit, but the gift the thumb brings to us is the ability to grasp, to hold, to use our hands in ways which other animals cannot. Another fundamental skill my little girl has lost. It’s not that the loss of these things makes my daughter any less of a human being, but I cannot help but believe that it does make her a little less of Hannah: the little girl, teenager, woman she could be. I cannot know if Hannah has hope, whether she is aware enough of the things she cannot do to hope that one day she will, although the way she looks at her brothers playing and running and talking, it is hard to believe that she is not hoping to join them one day. If hope gives her the same sense of purpose and drive and determination as it brings the rest of us, then I hope that she does have hope, and that one of these days my stubborn, cheeky, sparkling little girl will tell me that her name is Hannah.
RETT SYNDROME RESEARCH TRUST WEBSITE
by Kelly Rae Chi
Rett Syndrome doesn’t usually run in the family. Researchers led by Alessandra Renieri at the University of Siena in Italy encountered two exceptional cases: one pair of sisters with the same mutation in the Rett-causing gene MECP2, and a second pair with identical deletions within the gene.
Despite having the same mutations in MECP2, the sisters represented the clinical spectrum of the disorder. For each pair, one sister had classical Rett Syndrome—she was unable to speak or walk or use her hands—while the other had a milder form of the disorder (called Zapella) and could talk using short phrases, walk and retained some hand function. Researchers describe these four women and possible genetic reasons why the severities of their symptoms were so different, in a PLOS ONE paper published a few months ago.
It’s not surprising that girls with Rett Syndrome generally show a wide range of symptoms. That’s partly because a mutated copy of the MECP2 gene is located on only one of two X chromosomes in a female cell; the other copy is healthy. One X chromosome becomes inactive in each cell early in development. In rare cases when many cells express the healthy copy of MECP2, women show a milder form of Rett. In the new study, however, both pairs of women were similar in how many of their maternally or paternally derived X chromosomes were inactivated, suggesting that something else might explain the differing severities of their disease.
Hypothesizing that other genes could contribute to these differences, the scientists sequenced a small proportion of the women’s genomes (about 1%) that is thought to code for proteins. (This strategy, called exome sequencing, is a less costly and less burdensome in terms of data analysis compared with whole-genome sequencing, and in recent years it has been shown to identify previously unknown genes for rare, inherited disorders, such as Freeman-Sheldon syndrome.)
The team located 112 genetic variants on 108 genes that were exclusive to the women with classical Rett. A subset of these variations, about 10 to 20, is believed to be relevant to impaired protein function in Rett, based on what’s already known about them.
These genes are involved in a range of functions. Interestingly, both women with classic Rett have variants on at least six genes that have been previously linked to oxidative stress. (The two people with Zappella had variants on three.) In a follow-up study, Renieri’s group found that the women with classical Rett, but not the two with Zappella, showed molecular signs of oxidative stress compared with healthy controls. But the link between MECP2 mutations and oxidative stress is still unknown, the authors note.
The women with Zappella had exclusive variants in 80 genes, but none of these were shared by both. Some genes are linked to immune function, and the variants may be involved in protection from a more severe phenotype, says Renieri, a professor of medical genetics.
Although exome sequencing will continue to bear genetic clues on the variability of Rett Syndrome, the meaning of these variants will need further study, Renieri says. “I’m not sure now that all the variants we describe in the paper are relevant,” she admits. “In the next few years we will learn better how to interpret these results.”
Renieri’s group hopes to sequence the exomes of more people with severe and mild Rett Syndrome, to understand their genetic similarities and differences. It is easier to compare the genes of sisters because their genomes are 50% identical. But because sisters with Rett are so rare, they will need to compare unrelated patients, she says.
RETT SYNDROME RESEARCH TRUST WEBSITE
The following piece comes to us from the blog of a UK newspaper, The Independent. This powerful and poignant piece was written by Beth Whitley mother to Hannah who has Rett Syndrome. (3/15/2013)
::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::
Life with Rett Syndrome: ‘When my little girl was diagnosed, I had no concept how much things were going to change’
by Beth Whitley
I lost an old friend this week. Not in the idiomatic sense that he passed away, nor in the literal sense that I misplaced him in a crowded supermarket and never found my way back to him. Although, metaphorically, perhaps that’s exactly what happened: we lost each other in the crowded supermarket of life and by the time we realised we’d gone astray, there were just too many aisles and trolleys and shelves of tinned goods to find our way back. Of course, if I hadn’t been pushing a wheelchair maybe I’d have been able to keep up a bit better.





![photo[7]](https://rettsyndrome.files.wordpress.com/2014/11/photo7.jpg?w=223&h=300)
![photo[8]](https://rettsyndrome.files.wordpress.com/2014/11/photo8.jpg?w=223&h=300)
![photo[7]](https://rettsyndrome.files.wordpress.com/2014/11/photo71.jpg?w=223&h=300)